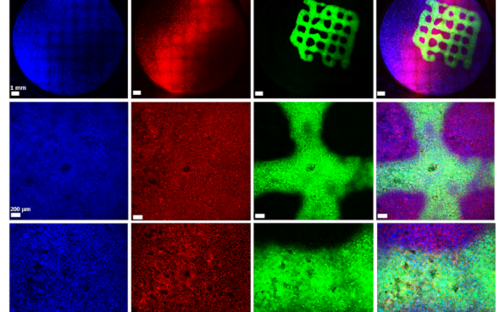
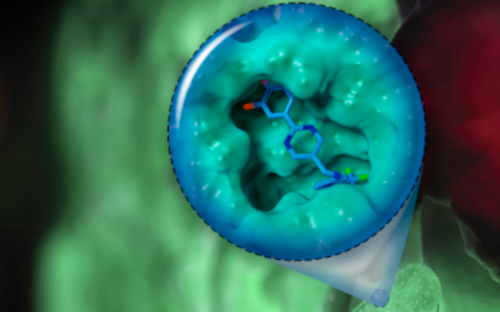
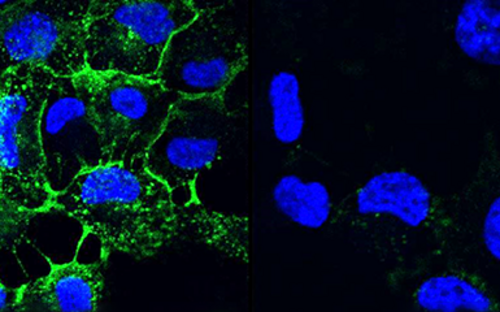
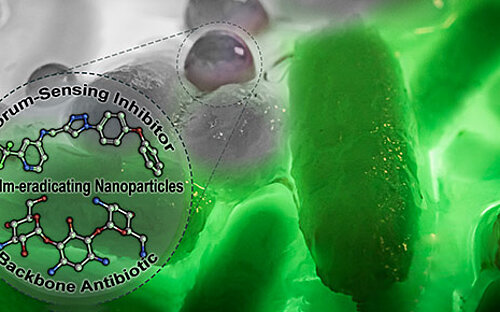
Biofilms – living in slime
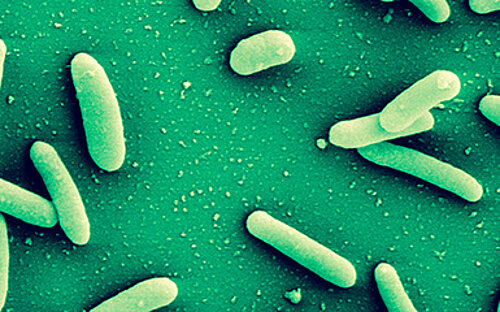
Covered by a thick slimy coating made of biopolymers, the bacteria shield themselves from the immune system and antibiotics. In Germany alone, some 100,000 infections annually are related to biofilms – with clinically relevant pathogens including pseudomonads, staphylococci, and streptococci.
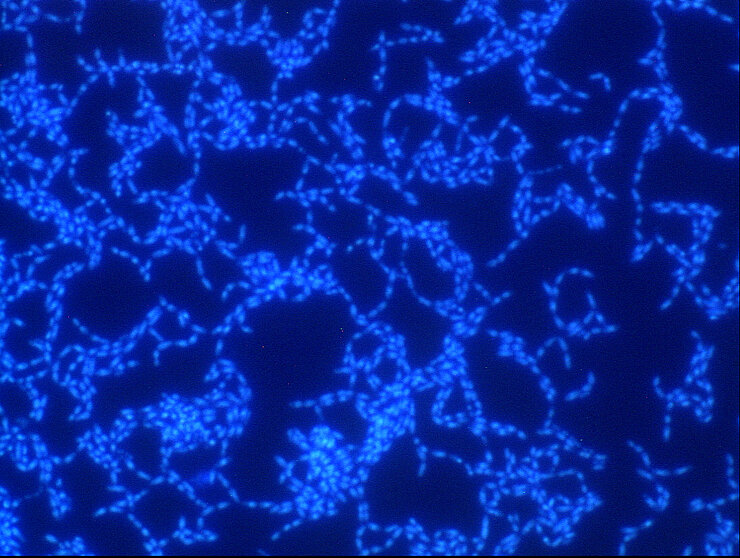
Bacteria are diverse – also with respect to their life form. On the one hand, they are capable of swimming around in an aquatic environment, e.g. a pool, a sink, blood, or some other body fluid. When swimming in such a way, they are loners and, depending on their individual virulence, cause more or less serious acute infections. On the other hand, they also build large communities that stick to surfaces.
They settle down, send out molecules, which tell other bacteria that a new bacterial colony was just founded, and coat themselves in a slimy matrix. They are in lively contact with each other via a chemical broadcasting system known as quorum sensing. In this state, they cause chronic infections that may lead to acute infectious episodes whenever individual bacteria are released from the biofilm community. Covered by a thick slimy coating made of biopolymers, the bacteria shield themselves from the immune system and antibiotics. In Germany alone, some 100,000 infections annually are related to biofilms – with clinically relevant pathogens including pseudomonads, staphylococci, and streptococci.
Scientists at the Helmholtz Centre for Infection Research are studying biofilms from several different angles. Their aim is to understand the principles underlying the biofilm communities and manipulate them. They are also searching for strategies to dissolve the biofilms – so that they can be attacked by the immune system and antibiotics.
Understanding biofilms
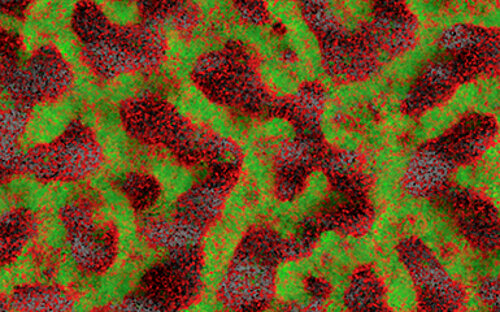
Biofilms are highly complex communities in which a wide variety of bacteria and fungi live, but not every biofilm is automatically pathogenic. How they behave depends on various factors and is controlled by chemical signals. Every day we brush them off our teeth and wipe them out of our sinks and immediately afterwards they come together again and reorganize themselves.
The biofilm always grows along
Biofilms on medical implants cannot be prevented: Whether stents, pacemakers or dental implants - the biofilm always grows with them. But perhaps they can be modified using suitable surfaces so that they do not harm the patient? Or is it possible to disrupt the bacteria's funk and thus dissolve their community? And does the patient have any influence on the biofilm? Are these communities even suitable as markers for diseases? Our scientists think: yes.
Fighting biofilms
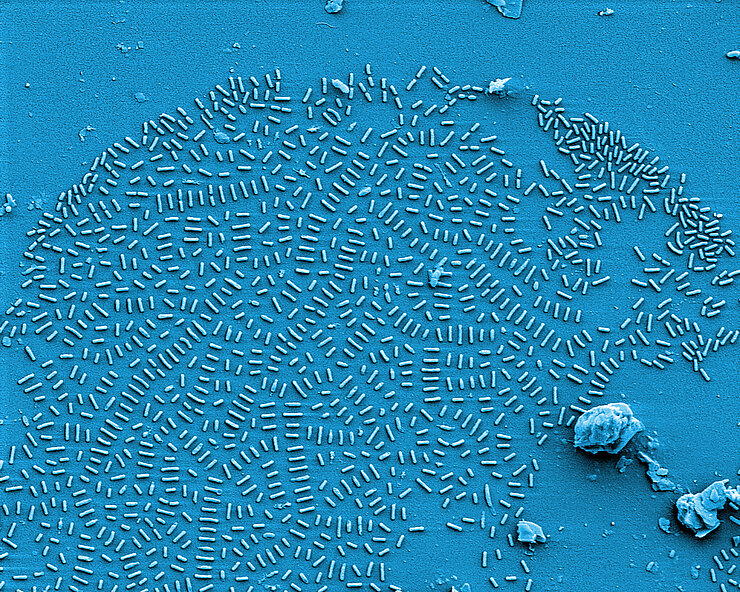
If pathogenic biofilms form in a seriously ill patient, the bacteria, which could cause a severe infection, have to be killed. One approach taken by HZI scientists as part of an interdisciplinary consortium is to dissolve the biofilm. The researchers are looking for natural substances that interfere with the bacterial communication system and signal the community to disintegrate. Then, the immune system and antibiotics can intervene and kill the bacteria.
One important consideration is that many of the clinically relevant pathogens have developed multi-resistance, caused through overuse of antibiotics during disinfection treatment of biofilms in medical everyday life. Trying to identify a suitable antibiotic for the patient often takes days, during which the already weakened patient is at the bacteria’s mercy. Therefore, our scientists are developing methods that work significantly faster than traditional microbiological techniques: They identify exactly those bacterial genes, which encode antibiotic resistance.
(jsg)
Involved research groups
-
Chemical Biology of Carbohydrates
 Prof Dr Alexander Titz
Prof Dr Alexander Titz -
Molecular Bacteriology
 Prof Dr Susanne Häußler
Prof Dr Susanne Häußler -
Drug Design and Optimization
 Prof Dr Anna K. H. Hirsch
Prof Dr Anna K. H. Hirsch