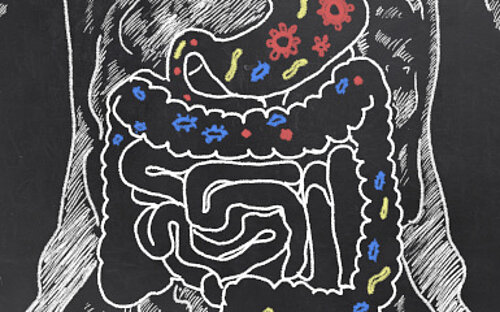
The microbiome – Only united we are strong
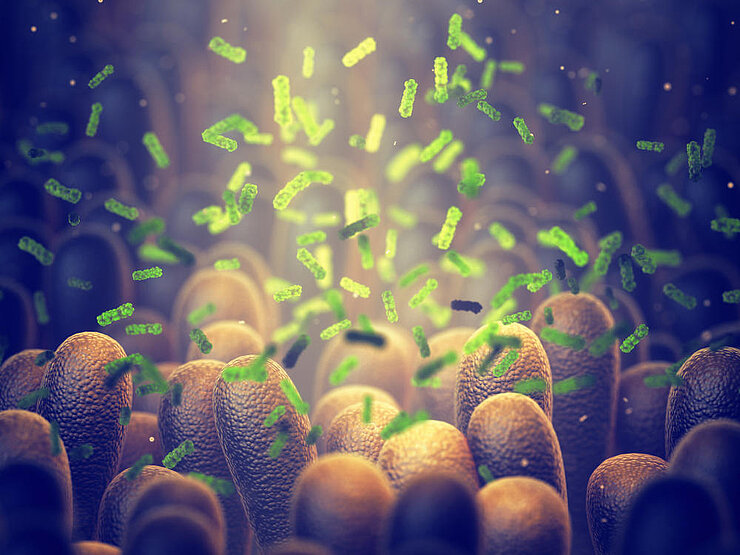
The microbiom makes an important contribution to our health, indeed the composition and the exact mode of operation are not known yet. Like a tropical rain forest, the microbiom is a complicated ecosystem. It only works whether all the different organisms collaborate perfectly.
Looking at our body in a mirror, we usually see only ourselves. The human being, the independent organism. What we usually fail to see, are the Allied forces in us and on us: Microscopically small allies that are with us at all times and support us and often defend us against enemies. Though invisible to us, they form a symbiotic community with us and neither of us thrives without the other. The billions of microorganisms, i.e. bacteria, Virusesand others, which colonise mainly our intestines, but also the skin and other parts of the body, are crucially important for us. Experts call this community the "microbiome".
As the host organism, we humans contribute a lion's share of approximately 99% to the total biomass, i.e. the weight of this community. But comparing the number of human body cells and the number of the much smaller microbes, things look different: For a long time, it was assumed that microbes outnumbered human cells by 10 to 1. New estimates indicate that microorganisms and host cells are roughly in a 1:1 ratio.
Intestinal bacteria support us in the process of digestion and even protect us from pathogens. The dense colonisation of the habitats on the intestinal wall leave no room for morbid pathogens. You might say the "good" bacteria defend us against the "bad" ones. In addition, they train our immune system. Studies have shown that the developing intestinal flora makes a crucial contribution to the formation of our immune system. Mice growing up in the absence of bacterial flora in their intestines, i.e. germ-free mice, have a strongly underdeveloped immune system later on.
The composition of the human microbiome is highly variable and is subject to influences for example of nutrition, immunocompetence and medications. We, as the Host, are usually at peace with our microbiome, but pathogens might join in. Pathogens start colonising us mainly when the microbiome is out of balance.
This kind of disturbance can be triggered by internal as well as by external factors. For example a treatment with antibiotics is directed mainly at killing bacteria that make us sick, but it will also affect, to some extent, the "good" bacteria that are present. The same can be observed in many human diseases, such as obesity, diabetes and inflammatory intestinal diseases. These diseases can be secondary to a disturbance of the microbiome, but they can just as well be what triggers the disturbance in the first place.
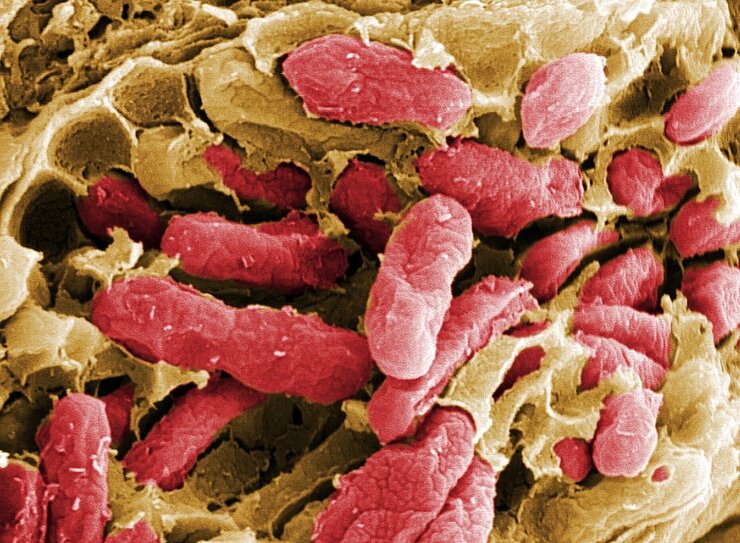
In fact, testing the microflora now allows conclusions to be made regarding the health status of the patient. Medical doctors can distinguish between a healthy and a disturbed community of microorganisms in the mouth, on the skin or in the genital area and make their diagnoses accordingly.
This shows that the microbiome makes an important contribution to our health, although its composition and exact function are not yet fully known. Similar to a tropical rain forest, the microbiome is a complex ecosystem. It works only if the various organisms interact perfectly. This is also the reason why it is so fragile.
The challenge in microbiome research is to understand this complex network in detail, to recognise the existing relationships and to decipher the interaction with the human body. One major obstacle to overcome in this research is that not even close to all organisms have been identified. This means that we often know how many types of bacteria belong to the microbiome, but are ignorant of the function of each individual species or of what it needs for survival.
The "Microbial Immune Regulation" research group at the HZI directed by Till Strowig investigates how these microbial communities affect infectious diseases and how they can be manipulated in order to treat diseases. They use well-defined model systems for this purpose - such as, for example, mice with a specific microbiome - and a broad range of methods. Using these tools, they analyse the interplay between the immune system of the host, the colonising microorganisms and the bacterial and viral pathogens. Understanding the underlying regulatory networks can lead to new medications or forms of therapy that can be used to treat or even prevent infectious diseases, which take millions of human lives each year.
Involved research groups
-
Anti-infectives from Microbiota
 Prof Dr Christine Beemelmanns
Prof Dr Christine Beemelmanns -
Bacterial Infection Ecology
 Dr Martin Jahn
Dr Martin Jahn -
Computational Biology for Infection Research
 Prof Dr Alice McHardy
Prof Dr Alice McHardy -
Dynamics of Respiratory Infections
 Prof Dr med. Hortense Slevogt
Prof Dr med. Hortense Slevogt -
Human-Microbe Systems Bioinformatics
 Jun Prof Dr Alexey Gurevich
Jun Prof Dr Alexey Gurevich -
Microbial Immune Regulation
 Prof Dr Till Strowig
Prof Dr Till Strowig -
Microbial Interactions and Processes
 Prof Dr Dietmar Pieper
Prof Dr Dietmar Pieper -
RNA Biology of bacterial infections
 Prof Dr Jörg Vogel
Prof Dr Jörg Vogel -
Host-Pathogen-Microbiota Interactions
 Prof Dr Alexander Westermann
Prof Dr Alexander Westermann