The discovery of antimicrobial compounds such as conventional antibiotics has transformed medicine, allowing for the treatment of infections that were once deemed untreatable. However, the development pipeline for new agents has slowed, while the improper use of existing antibiotics has fueled the emergence of antibiotic resistance. Consequently, there is a growing need for new means to eradicate pathogens.
Antimicrobial agents of the future
CRISPR-Cas systems, adaptive immune mechanisms bacteria employ to defend against viral invasion, offer a distinct solution through their capacity to selectively eliminate microbes based solely on genetic sequences. Yet to-date, systematic studies to assess the efficacy of these CRISPR antimicrobials, especially across different nucleases, target sites, and bacterial strains, have been lacking. Addressing this gap, an international team led by the Helmholtz Institute for RNA-based Infection Research (HIRI), a site of the Braunschweig Helmholtz Centre for Infection Research (HZI) in cooperation with the Julius-Maximilians-Universität Würzburg (JMU), has now undertaken the first comprehensive interrogation of these novel agents. Their research focuses on Klebsiella pneumoniae, a bacterium notorious for its association with antibiotic resistance.
“Klebsiella pneumoniae offers a particularly compelling case study given that it comprises numerous strains with varying virulence and resistance characteristics,” says Chase Beisel, head of the RNA Synthetic Biology department at the HIRI and professor at the JMU Medical Faculty. He spearheaded the international study in collaboration with researchers from the Institut Pasteur in Paris, France, Tel Aviv University in Israel, HZI, and the University of Toronto in Canada. Besides coming from four different countries, the team combined expertise in CRISPR technologies, Klebsiella bacteria, bacteriophage delivery, high-throughput screens, and machine learning needed to conduct a study of this scale. Their findings were published today in the journal Nucleic Acids Research.
A different strain, (sometimes) a different effect
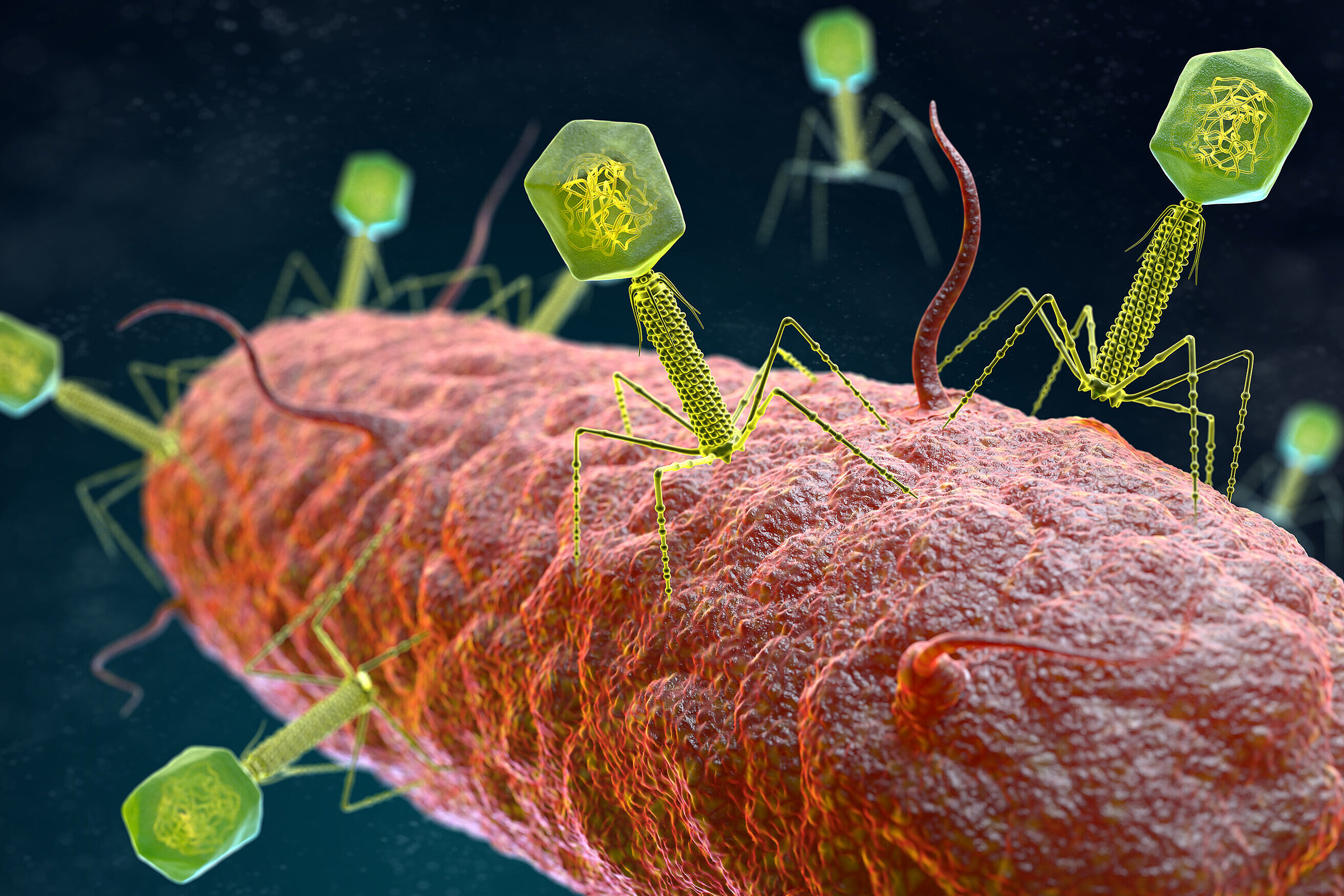
CRISPR-Cas systems use a sophisticated defense mechanism: A CRISPR ribonucleic acid (RNA) helps to detect regions of a foreign genome, such as DNA or RNA, for targeted cleavage. Subsequently, the CRISPR-associated (Cas) nuclease cuts its target akin to a pair of molecular scissors. The scientists found that different CRISPR nucleases exhibit widely varying efficacies. In their experiments, nucleases targeting DNA showcased superior performance compared to those focusing on RNA.
Moreover, different types of K. pneumoniae showed variation in their sensitivity to a CRISPR antimicrobial, despite employing identical nucleases to target identical sites. Elena Vialetto, the study's first author and former PhD student in the Beisel lab, remarks: “The variable antimicrobial activity between related bacteria was surprising given the use of the same CRISPR constructs. We attributed this difference to the folding of the CRISPR RNAs that guide DNA targeting.” Chase Beisel further underscores: “This study is the first to demonstrate that the antibacterial effectiveness can vary even between related strains.”
To explore features that could improve targeting across various strains, the researchers conducted a genome-wide screen in different K. pneumoniae types. This effort yielded design principles and parameters for possible CRISPR antimicrobials and facilitated the training of an algorithm to forecast their efficiency.
Phages as Trojan horses
The team also ventured into the next stage of active agent development, namely delivery. The researchers used bacteriophages as vehicles for the CRISPR antimicrobials, which they equipped with modified tail fibers to increase the range of the CRISPR cargo.
This study lays the foundation for the further development of CRISPR as a means to prevent or treat antibiotic-resistant infections. “We hope this work will bring greater visibility to the use of CRISPR as a tailored-spectrum antimicrobial in the ongoing fight against antibiotic resistance,” Beisel concludes.
Funding
The study was supported by the Joint Programming Initiative on Antimicrobial Resistance, the Bavarian State Ministry for Science and the Arts through the research network bayresq.net, and the European Research Council (Grant to Udi Qimron).
Original publication
Vialetto E, Miele S, Goren MG, Yu J, Yu Y, Collias D, Beamud B, Osbelt L, Lourenço M, Strowig T, Brisse S, Barquist L, Qimron U, Bikard D, Beisel CL: Systematic interrogation of CRISPR antimicrobials in Klebsiella pneumoniae reveals nuclease-, guide- and strain-dependent features influencing antimicrobial activity. Nucleic Acids Research (2024), DOI: 10.1093/nar/gkae281