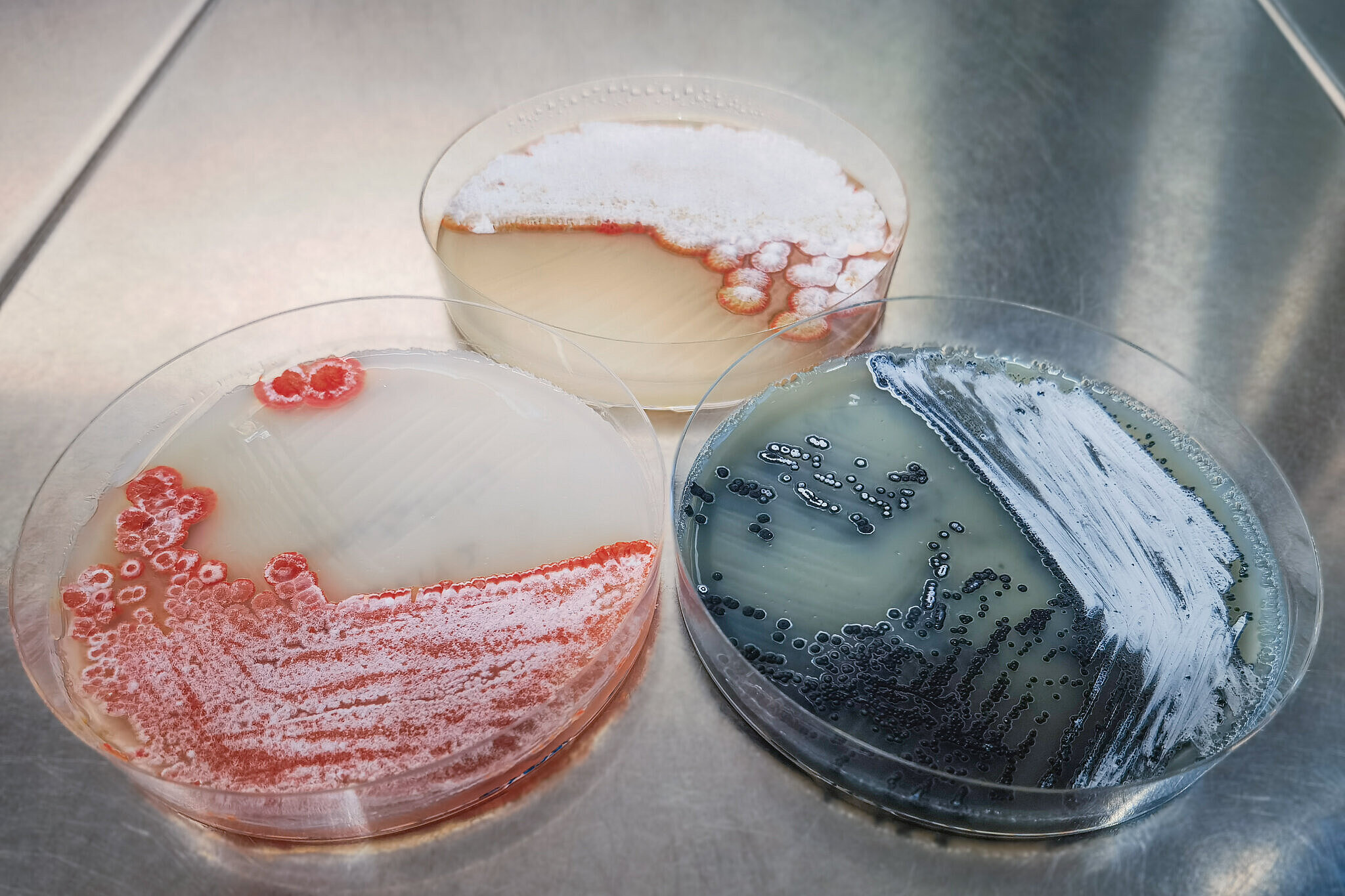
The more frequently antibiotics are used, the faster pathogens evolve mechanisms to evade their effects. This leads to resistant pathogens against which common antibiotics are no longer effective. To ensure that effective treatments for bacterial infections remain available in the future, antibiotics that target different bacterial structures than currently approved drugs are essential. One such candidate was discovered by HIPS researchers back in 2008 in the soil bacterium Sorangium cellulosum: the natural product class of chlorotonils. These compounds exhibit strong activity against hospital pathogens Staphylococcus aureus and Enterococcus faecium, as well as the malaria pathogen Plasmodium falciparum, and act via a previously unknown mechanism. The HIPS is a site of the Helmholtz Centre for Infection Research (HZI) in collaboration with Saarland University.
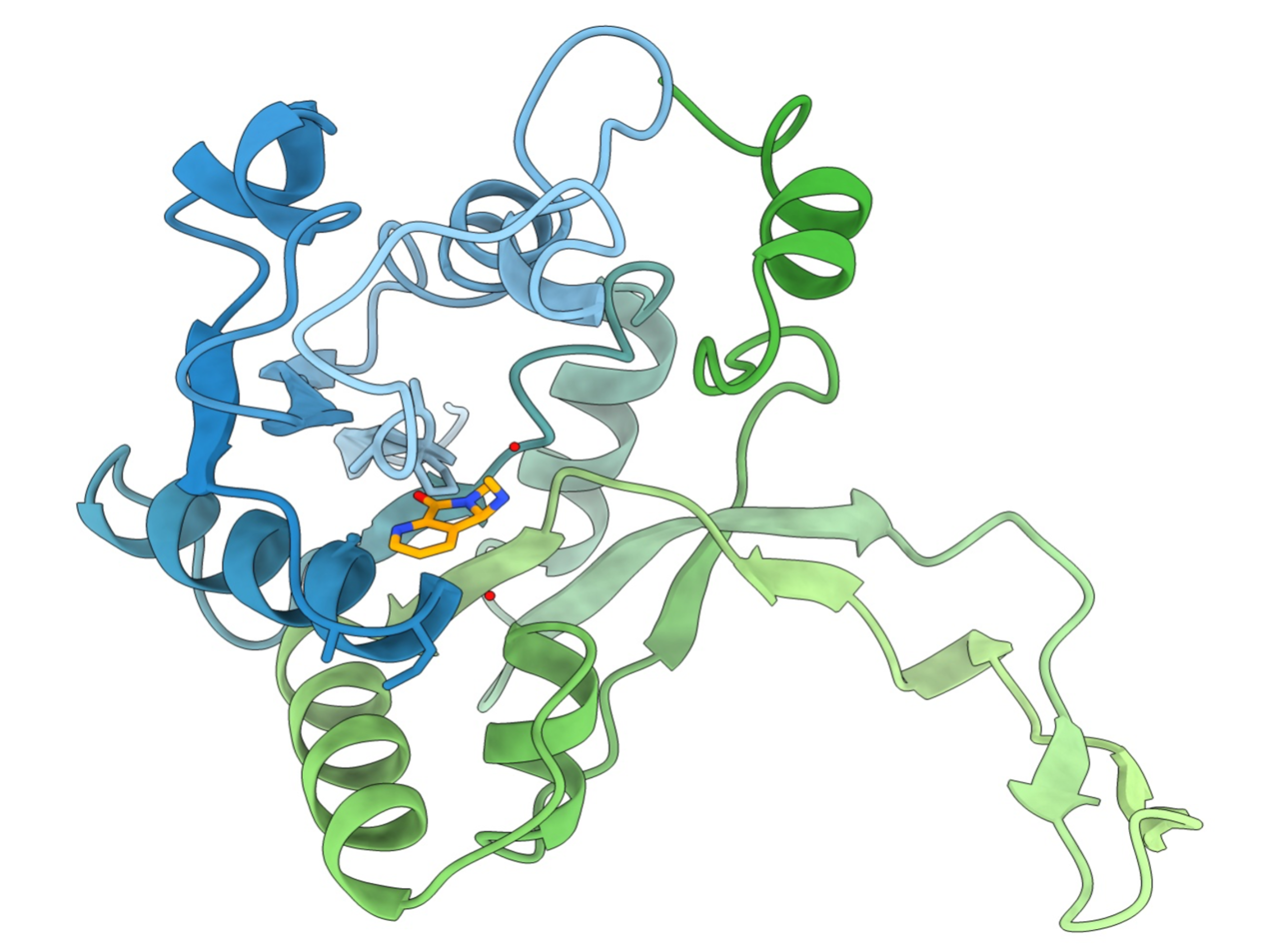
In the newly published study, researchers led by Dr. Jennifer Herrmann and Prof. Rolf Müller uncovered the novel mode of action of chlorotonils. They demonstrated that chlorotonils attack bacterial pathogens with a combined approach, unlike most antibiotics. On one hand, they bind to membrane lipids, destabilizing the bacterial membrane. Additionally, they inhibit two enzymes involved in cell wall and protein synthesis. First author Dr. Felix Deschner, a postdoc in Müller’s department “Microbial Natural Products”, explains exactly how chlorotonils exert their effect: “When chlorotonil binds to the cell membrane, potassium ions can leak uncontrollably out of the cell. This throws the cell’s internal environment off balance – the membrane’s electrical potential changes, osmotic pressure drops rapidly, and essential cellular processes are disrupted.” In combination with the inhibition of the phosphatase YbjG and the methionine aminopeptidase MetAP, the bacterial cell’s functions are so severely impaired that cell death ultimately occurs.
“Initially, we had promising efficacy studies, but the target structure and exact mode of action were unclear,” says Deschner. To address these questions, the researchers conducted extensive experiments and created a “profile” of the molecule. “Through this, we discovered that chlorotonils bind directly to lipids, thereby influencing the membrane potential. This was unexpected, as it represents a rarely observed antibiotic mechanism,” Deschner explains. The alteration of membrane potential results in immediate activity, which also explains chlorotonils’ rapid bactericidal effect. Their direct interaction with membrane lipids also makes it more difficult for bacteria to develop resistance mechanisms against chlorotonils. If an antibiotic targets a specific enzyme, bacteria can either produce more of it or structurally alter it to protect themselves. These options don’t apply to lipids. Only through mutations in the lipid efflux system, which controls the composition of the cell membrane, were more resistant bacterial strains identified. Understanding the resistance mechanism against an antibiotic is crucial for developing strategies to counteract it – for example, through combination therapies or structural modifications of the compound.
“Our findings show that chlorotonils pursue an entirely new mode of action and simultaneously target multiple critical structures in the bacterial cell,” says Herrmann. “This makes them potential game-changers in the fight against multidrug-resistant pathogens and opens up opportunities to systematically search for other agents with similar mechanisms.” The researchers are currently working on optimizing the efficacy and safety of chlorotonils. In parallel, under the GO-Bio initial program, they are developing chlorotonils into a drug for the treatment of malaria.


![[Translate to English:] 3D-kontrastierte Oberflächenstruktur von LasB (beige) mit einem Inhibitor der 3. Generation (dunkelblau), der Zink (grau) bindet.](/fileadmin/_processed_/8/b/csm_LasB_Foto_b7734ae7a4.png)