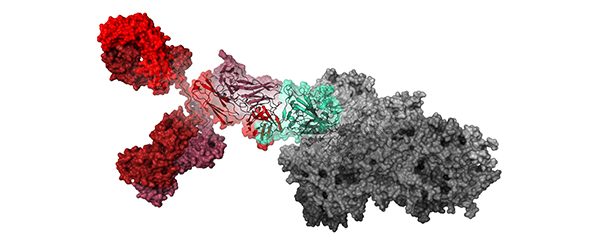
In the first step, the researchers isolated the genetic information of immune cells, which are producing anti-SARS-CoV-2 antibodies, from blood samples from local convalescent COVID-19 patients. From the collected antibody genes, they constructed a phage display library. Nearly 200 unique human antibodies binding to the Receptor Binding Domain (RBD) in the spike protein of SARS-CoV-2 were discovered in the test tube using the phage display approach. In tests performed at the BSL-3 facilities of the Helmholtz Centre for Infection Research (HZI), 30 of these antibodies efficiently blocked the infection of cells with patient-isolated SARS-CoV-2. The antibody COR-101 showed the best neutralization activity. The efficacy of the antibody was confirmed in transgenic mice, which express the human ACE2 receptor, and syrian hamsters. COR-101 efficiently reduced or completely eliminated the virus loads in the deep lung. The research teams determined the atomic structure of the antibody in complex with the RBD and showed that the antibody directly and broadly blocks the interaction of the RBD with the human ACE2 receptor. Significantly, COR-101 binds and inhibits the spike proteins of most recently emerging SARS-CoV-2 variants, including the variants first identified in India (B.1.617.1 and .3). New additional data, generated after publication in Cell Reports, indicate that even the most recent WHO variant of concern, B.1.617.2 („Delta“), is also efficiently inhibited.
In contrast to the antibodies, which got an emergency use authorization in the EU and are designed for the treatment of non-hospitalized patients with mild symptoms, the antibody COR-101 was developed specifically for the treatment of COVID-19 patients with moderate to severe symptoms, who are hospitalized and need urgent medical support. The researchers achieved this by modifying a part of the antibody to avoid the activation of the immune system, thereby preventing adverse reactions in the patients due to overshooting immune responses.
Prof Michael Hust (TU Braunschweig), the lead senior author of this study and co-initiator of the CORAT initiative, comments: “The COR-101 antibody development project was only possible because of the close joint collaboration of our group with the YUMAB GmbH and the HZI. I’m optimistic, that this antibody will save many lives of COVID-19 patients.”
Dr Maren Schubert (TU Braunschweig), co-senior author of this study, says: “We are proud to see the results of the research and development we started in February 2020 with the first production of SARS-CoV-2 proteins in insect cells.”
Prof Stefan Dübel (TU Braunschweig), inventor of the antibody phage display technology, which was used to discover the antibody, and initiator of the CORAT initiative, emphasized: “We have demonstrated that we can develop effective therapeutic antibody drug candidates in a very short time. This knowledge will allow us to act faster and more specific in the next pandemic situation.”
Dr Joop van den Heuvel, structural biologist at HZI, adds: “We are fascinated about the mode of action of this unique antibody.”
Prof Luka Čičin-Šain, head of the department “Viral Immunology” at HZI: “COR-101 shows the excellent capability of the Braunschweig region for translational infection research.”
Dr André Frenzel, CSO of YUMAB and CORAT: “We are looking forward to see the clinical trial data of COR-101, an antibody which fills the current gap in the therapy of COVID-19 patients.”
Original publication
Bertoglio, F., Fühner. V., Ruschig, M., Abassi, L., Heine, P.A., Klünemann, T., Rand, U., Meier, D., Langreder, N., Steinke, S., Ballmann, R., Schneider, K.-T., Roth, K.D.R., Kuhn, P., Riese, P., Schäckermann, D., Korn, J., Koch, A., Chaudhry, M.Z., Eschke, K., Kim, Y., Zock-Emmenthal, S., Becker, M., Scholz, M., Moreira, G.M.S.G., Wenzel, E.V., Russo, G., Garritsen, H.S.P., Casu, S., Gerstner, A., Roth, G., Adler, J., Trimpert, J., Hermann, A., Schirrmann, T., Dübel, S., Frenzel, A., Van den Heuvel, J., Čičin-Šain, L., Schubert, M. & Hust, M.: A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients is binding to the ACE2-RBD interface and is tolerant to most known RBD mutations. Cell Reports 2021; doi: 10.1016/j.celrep.2021.109433
About the study
The study was conducted in the core research area “Infections and Therapeutics” at the TU Braunschweig. Mice and hamster were used for the studies. The animal experiments took place at HZI and FU Berlin and were conducted under strict safety and animal protection regulations.
Further Links
About the Corona Antibody Team
About CORAT Therapeutics (clinical development)