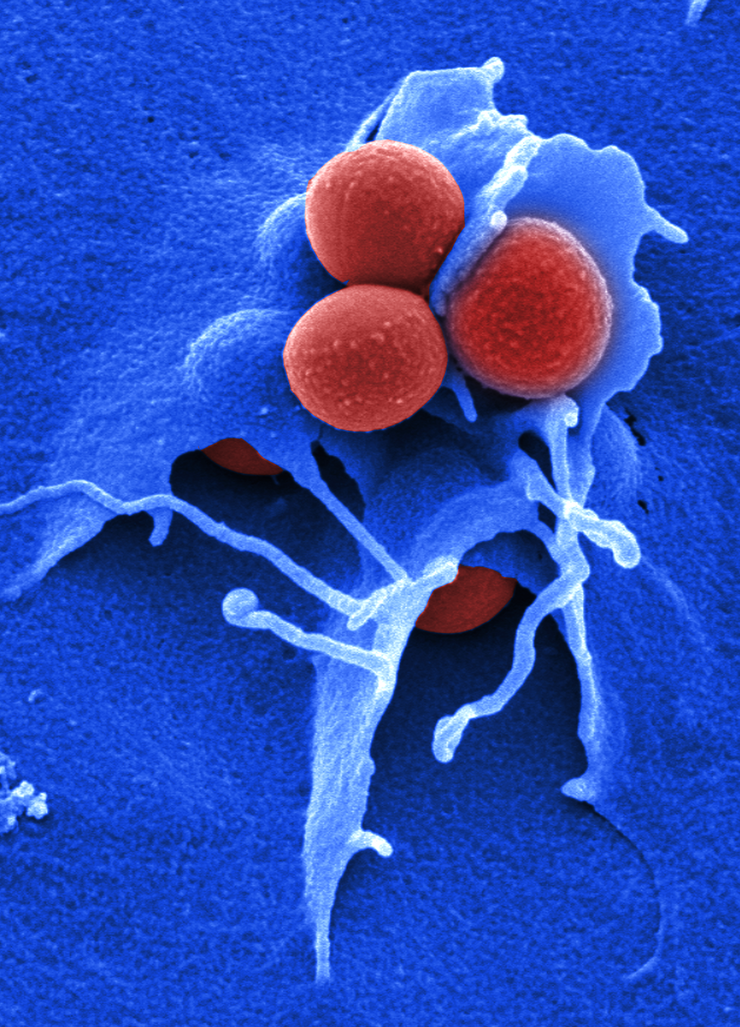
Prof Rolf Müller, who is the speaker of the research topic “Anti-infectives” at the Helmholtz Centre for Infection Research (HZI) and the Executive Director of the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) in Saarbrücken, summarises the status quo as follows: “Antibiotic resistance is a global problem. According to estimates, more fatalities will be caused by antibiotics resistances than by cancer by the year 2050. Unfortunately, little has been done against the multi-resistant pathogens in the past ten years, and these are causing the greatest problems in our hospitals to date. Accordingly, there is an urgent need to develop new effective medications.”
Racing against pathogens
Hospitals in focus – backlog in diagnostics
Prof Susanne Häußler, who is a medical doctor and the head of the “Molecular Bacteriology” department at the HZI, also considers the status quo to be very critical: “The number of multiresistant pathogens in hospitals keeps growing, while the number of newly approved medications is dropping drastically.” According to Häußler, medical microbiology still needs to do its homework, since the common resistance tests for bacteria in clinical settings are based exclusively on the growth in the presence of antibiotics. And this may take too long. Moreover, the researchers obtain no information concerning the resistance mechanisms and the clonal identity of the pathogens. “For this reason, we are working on molecular diagnostics: We isolate bacterial genetic material – RNA and DNA – and determine genetic markers of resistance. We also do accurate typing,” says Häußler. “This allows us to monitor the spread of multi-resistant strains faster and more specifically. Medical doctors can then initiate the appropriate prophylactic and therapeutic measures.”
Antibiotics research – unattractive to pharmaceutical companies
In most cases, only antibiotics that are not very commonly used yet can help against resistant pathogens. But these new medications are either very expensive or they don‘t even exist yet. Only a single new class of agents against Gram-negative pathogens has been developed since 1987 – and this class includes a single agent. In addition, pharmaceutical companies rarely invest in the development of new antibiotics anymore, because these products can be used only infrequently and only for a short period of time, which makes them not very profitable. As recently as in July 2018, the pharmaceutical company Novartis abandoned its antibiotics and infection research. “Without a major change in medical research and development, diseases that can be treated today may become incurable in just a few years,” says Rolf Müller. “This gap in research must now be filled by extramural research facilities such as the HZI and other institutions that search for promising candidate agents and optimise the chemical compounds as much as possible for subsequent commercial development by pharmaceutical companies.”
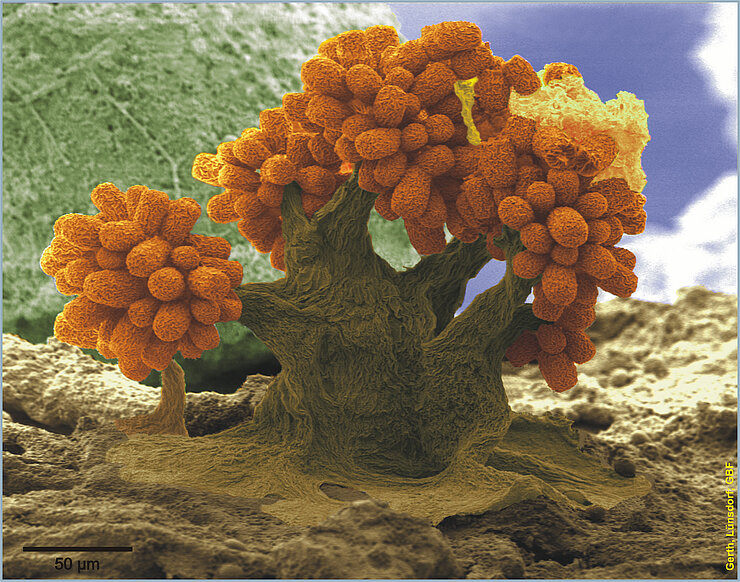
The hunt for new antibiotics
Nature is the best resource for new agents. “Approximately 80 per cent of all antibiotics are natural products. Fungi are a virtually inexhaustible source of new bioactive secondary metabolites. They produce antibiotics under natural conditions to prevail over bacteria and to survive their attacks,” says Prof Marc Stadler, who is the head of the “Microbial Drugs” department at the HZI. Soildwelling myxobacteria are known to produce certain secondary metabolites, but have not yet been studied in much detail. To date, the collections of the HZI and the HIPS have accumulated more than 11,000 strains of myxobacteria. In their search for new anti-infective agents, the researchers analyse the genetic material of the microorganisms, which allows them to estimate whether or not there is any potential for the production of useful agents. If considerable amounts of the substance can be produced, the researchers use various assays to test for interesting effects against problematic bacterial and fungal germs and cancer cell lines. In the end, all substances are gathered in a natural product library and can therefore be checked for further effects at any time. Aside from fungal and myxobacterial compounds, the library also contains numerous samples from the actinobacteria, i.e. the organism group from which historically the majority of antibiotics have been derived. Müller‘s team at the HZI and the HIPS already discovered new candidate antibiotics amongst these substances, for example the cystobactamides, which kill mainly Gram-negative pathogens that are particularly difficult to control. A proof of concept concerning the efficacy in infections in an animal model has already been documented. In collaboration with their colleagues from the HZI in Braunschweig and partners from the pharmaceutical industry, the researchers also optimised an agent called griselimycin, which is effective against the tuberculosis pathogen, and they even elucidated its mechanism of action. This agent showed excellent activity against the tuberculosis pathogen in an animal model and its activity is directed against all multi-resistant pathogens known at this time.
But substances that are very effective against bacteria are not automatically also well-suited for application in humans. It is often a long and rocky road in medicine – and only few candidates end up being successful. As if this was not difficult enough anyway, they must be amenable to being produced in sufficient quantities in biotechnological processes. In many cases, the biotechnology platform of the HZI has managed to provide sustainable processes to access the desired molecules in multi-gram scale, but sometimes it is not feasible in a cost-efficient manner. This is where the synthetic biology and medical chemistry come into play, as they replace the often very complex structures of these natural products by simpler molecules that are easier to optimise. These can then be adapted further, for example in order to attain better tolerability, higher stability in circulating blood or higher effective levels at the site of infection.
Disarming pathogens instead of killing them

Besides the development of new antibiotics, alternative strategies to counteract antibiotic-resistant pathogens are currently being investigated. Among them, the anti-virulence approach has gained popularity in recent years: It aims at disarming pathogens rather than killing them. Dr Eva Medina, head of the “Infection Immunology” research group at the HZI, searches for agents that can specifically interfere with the activity of factors produced by pathogens to cause disease – so-called virulence factors. These include proteins used by pathogens to penetrate into host cells or to defend themselves against the immune system. “The major advantage of this approach is that the bacteria stay alive but disarmed and therefore they are more easily eliminated by the immune system. As a result, the development of resistance against anti-virulence agents will be minimised as selection pressure that favors the selection of resistant bacterial subpopulations is anticipated to be weaker than in the case of traditional antibiotics.“ This makes the development of anti-virulence agents more attractive for pharmaceutical industry since their effectiveness will not be lost and can therefore be used in the clinic for longer periods of time. One limitation of the anti-virulence therapies is that they are highly specific and only work against the target pathogen. This requires a rapid and very accurate diagnostic of the infecting agent – even in patients with a life-threatening disease – that can result in therapeutic delay. Furthermore, based on experimental infection models, the team of Eva Medina has recently reported that the expression of virulence factors by pathogens is strongly influenced by the genetic configuration and the immune system of the individual host. “Most probably, the anti-virulence strategy also needs to be tailored to individual patients,” Medina says.
Innovative approach – programmable RNA antibiotics
Today‘s antibiotics usually are effective on a broad range: They attack either Gram-negative or Gram-positive bacteria and, doing so, destroy many harmless or useful microorganisms as well. But in certain infectious diseases, it may be necessary to eliminate one specific type of bacterium in the microbiome of the patient – e.g. in the intestinal flora. Prof Jörg Vogel, who is the director of the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg, pursues a novel research approach based on RNA molecules, which are copies of the genetic information. RNA molecules determine all kinds of processes in the cell and are also associated with many diseases. Vogel aims to drive back the pathogen and to better protect the host from the infection to the benefit of the patient. In both cases, RNA molecules play an important role, for example when pathogens check their surroundings to decide on the perfect moment for their attack on the host. “RNA molecules can be used to develop programmable drugs and to use them specifically against a certain type of bacterium, unlike traditional antibiotics. Other types of bacteria that are useful to humans stay unharmed,” Vogel says. “Using a method we developed, we can monitor in detail, which genes of a pathogen are switched on or off during an infection and what happens in the host afterwards. These insights present us with novel approaches for therapies.”
The major focus of the RNA researchers currently is on the pathogen called Fusobacterium nucleatum, which usually is present in the oral cavity, but also gets into the intestines when saliva is swallowed. Approximately seven years ago, it was found that fusobacterial attach themselves to precursor cells of intestinal cancer. “Although the bacteria do not seem to be a causative factor in the formation of intestinal cancer, they reduce the treatment opportunities drastically by inactivating the chemotherapeutic agents,” Vogel says. “With a programmable antibiotic, we might be able to specifically eliminate just the fusobacteria.” Currently, there are still many open questions concerning programmable antibiotics, but their efficacy has been confirmed on principle, and Vogel thinks they have great potential also in the correction of diseaserelated changes of the intestinal flora.
Author: Susanne Thiele
Published: November 2018