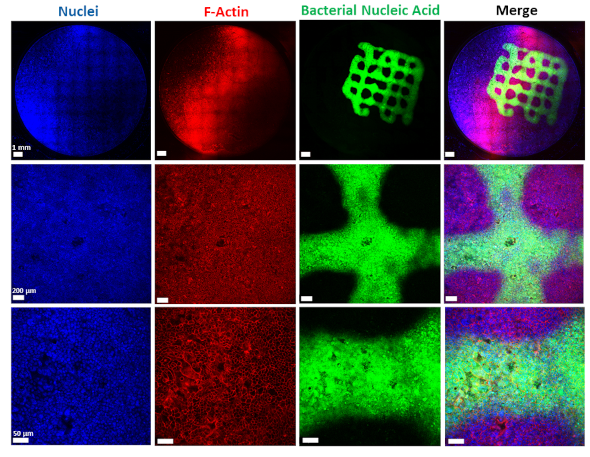
To develop new therapeutics against infections, researchers depend on laboratory models that allow them to simulate and study the infection process. Such models are essential, especially in the early testing and development of active substances, in order to keep the number of necessary animal experiments as low as possible. In the case of biofilm-associated infections, these models are very complex, since with the biofilm, in addition to human cells and the pathogens, another component comes into play that must be reproduced as realistically as possible. A team led by Claus-Michael Lehr, head of the Department of Drug Delivery across Biological Barriers at HIPS and Professor of Biopharmacy and Pharmaceutical Technology at Saarland University, has now succeeded in developing and characterising such a model system. HIPS is a site of the Helmholtz Centre for Infection Research (HZI) in cooperation with Saarland University.
In the published study, the bacterial cells, including the biofilm, are placed on a layer of lung epithelial cells by a special 3D printer. This so-called "bioprinting" is a complex process that requires an ink with special properties. "The development of a biofilm infection model is not trivial as the rapid growth of bacteria and the release of toxins can easily lead to premature death of the lung cells. As a result, preserving the biofilm in such a system requires a very controlled environment," explains Claus-Michael Lehr. "We optimised our 3D-printed biofilms to be very close to a native biofilm. A major challenge was that the artificial biofilms maintain their shape after washing off the excess bioink and do not have a toxic effect on the underlying lung cells. Both of these challenges have led to encouraging results with the model developed." To test biocompatibility with human cells, the biofilms were printed onto human bronchial epithelial cells. The constructs produced were evaluated using fluorescence and electron microscopy, among other methods, and gradually optimised. Also the sensitivity of the bacteria in the biofilm to clinically used antibiotics was investigated. The printed biofilms led to a similar protection of the bacteria against treatment with antibiotics as native biofilms and are therefore ideally suited to simulate a respective treatment.
"Our method can now be used to analyse several aspects of a biofilm-associated infection at once, including morphology, antibiotic sensitivity or changes in metabolism," says Samy Aliyazdi, PhD student in Claus-Michael Lehr's department and first author of the study. "Using 3D bioprinting, we were able to generate a robust human-based in vitro model that we now plan to use for the development of novel anti-infectives."
Text: Dr Alwin Hartman and Dr Yannic Nonnenmacher
Original publication
Aliyazdi S., Frisch S., Hidalgo A., Frank N., Krug D., Müller R., Schaefer U.F., Vogt T., Loretz B. & Lehr C.-M. 3D Bioprinting of E. coli MG1655 Biofilms on Human Lung Epithelial Cells for Building Complex In Vitro Infection Models. Biofabrication, 2023. DOI: 10.1088/1758-5090/acd95e