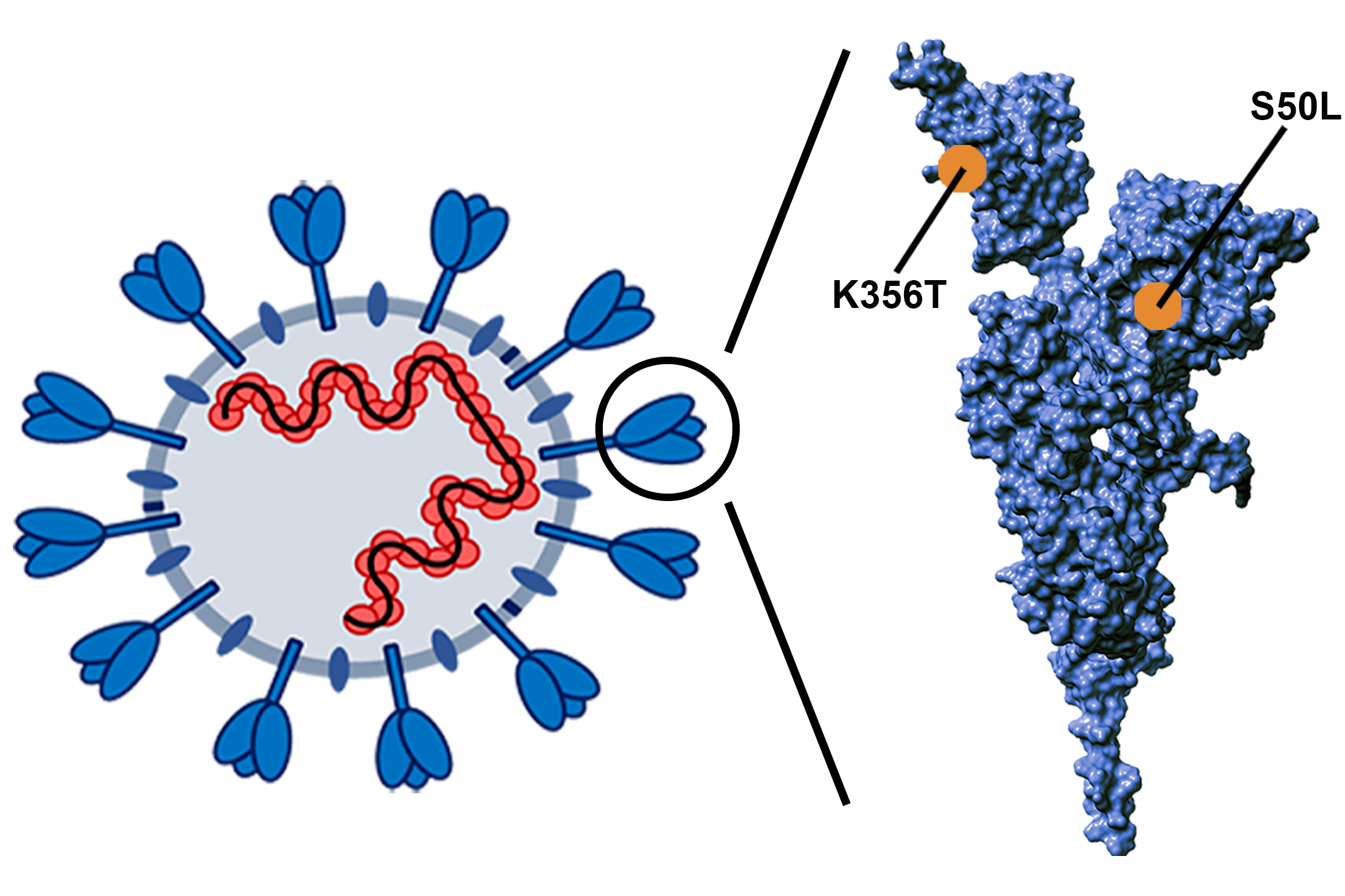
Mutations in the genome of viruses or bacteria occur every time the genetic material is replicated and rarely have a positive effect. If they do, they give the pathogen new characteristics. One example of these are the so-called “escape mutations”, which enable a virus to evade defense by the host organism's immune system. Other mutations can increase infectivity. If several of such mutations occur simultaneously, their combined effect usually is just the sum of the effect of the individual mutations. But that is not always true.
“This phenomenon by which pairs of mutations act synergistically is called an epistatic interaction,” says Prof Marco Galardini, head of the RESIST research group “Systems Biology of Microbial Communities” at the Institute of Molecular Bacteriology at TWINCORE. The bioinformatician is leading the project, which also involves researchers from other departments at TWINCORE and the department “Viral Immunology” led by Prof. Luka Cicin-Sain at the HZI in Braunschweig.
“Both escape mutations and increased infectivity were observed in the Omicron variant of SARS-CoV-2,” says Galardini. “Epistatic interaction can enable pathogens to acquire favourable properties even though the individual mutations are deleterious.” Galardini's team wanted to get to the bottom of this unusual finding. Most of the bioinformatical work was performed by Gabriel Innocenti, a visiting researcher in the lab in 2022, and now a PhD student at the Medical University of Vienna.
The researchers benefited from the fact that no other pathogen has as much sequence data as the SARS-CoV-2 coronavirus. “We were able to work with 15 million genome sequences that were collected worldwide during the pandemic,” says Galardini. “We were particularly interested in how early this effect could be detected or whether it could even be predicted based on the genome sequences”, Innocenti adds. In their computer simulations, the scientists were able to demonstrate the prediction of a known epistatic interaction with just seven genome sequences.
The research team was then able to prove in laboratory experiments that the viruses actually enter into the interactions predicted in the model. They co-operated with partners from the RESIST Cluster of Excellence. “The viruses behave in exactly the same way in infected cell cultures,” says Maureen Obara, PhD student at the Institute for Experimental Infection Research at TWINCORE. “We were able to confirm Marco's team's simulations with our experiments.”
Galardini is primarily researching the genetic properties of bacteria, for example how salmonella becomes resistant to antibiotics. “However, there are only one million sequence datasets for salmonella, which for sure is a very large number,” he says. “But the enormous number of coronavirus sequences has opened up a whole new field of research for us.”
In future, he also wants to apply his model to bacteria. Sequence data from the clinic could then be analysed to determine whether epistatic interactions can contribute to certain antibiotics no longer working. “We want to develop a warning system for the clinic,” says Galardini. He can also draw on the expertise of the other research groups in the interdisciplinary RESIST network.