Your work describes a new treatment option against Pseudomonas aeruginosa. Why is this bacterium so dangerous and why is there a need for new treatment concepts?
J. Haupenthal: "This pathogen plays an a major role in infections of the lungs, wounds and eyes and is mainly transmitted in hospitals. During infection, the bacterium produces a whole range of toxins, some with tissue-damaging properties, often with serious consequences for patients. Since the emergence of antibiotic resistance is playing an increasingly important role, the WHO classifies antibiotic-resistant "priority pathogens" into three severity levels. P. aeruginosa, as a priority 1 pathogen, is among the most critical of the three groups because it has become resistant to a number of antibiotics, including carbapenems and cephalosporins."
How did you find a molecule that can block the virulence of P. aeruginosa and how did you optimise it?
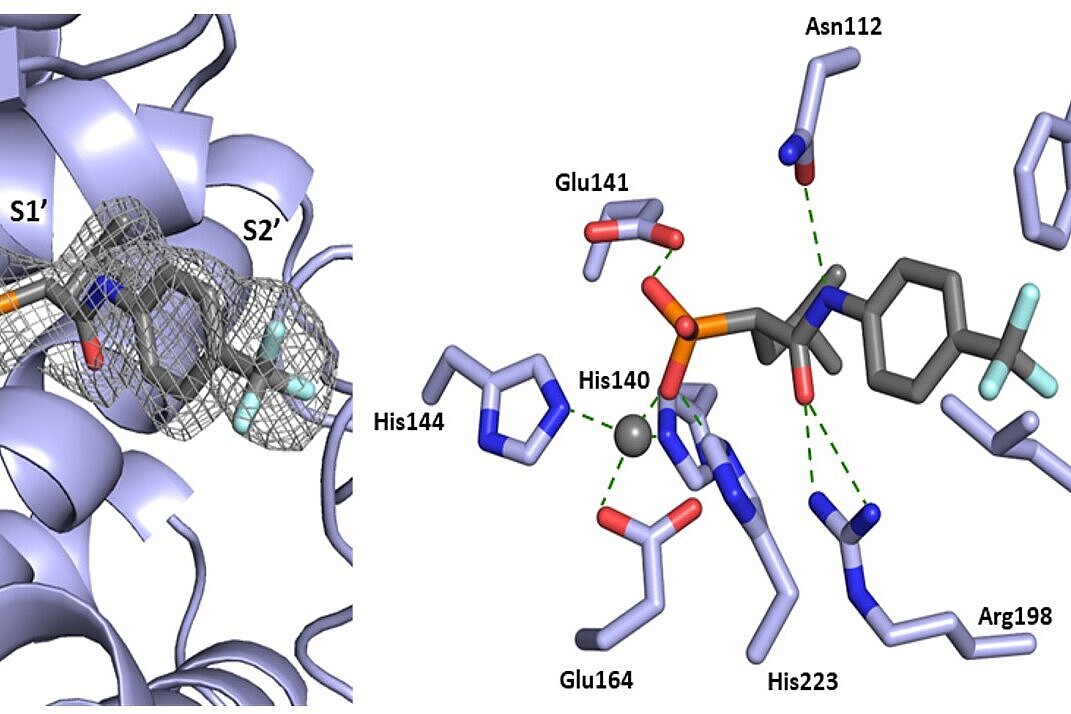
J. Haupenthal: "First, we focused on the protein LasB as a known and promising target. We purified LasB and looked for compounds that could inhibit its enzymatic activity. From the most promising hit, we progressively synthesised about 500 derivatives. The rational design of our new molecules was based on structure-activity relationships and high-resolution co-crystal structures of our compounds with LasB. This allowed us to get a very accurate picture of how our compounds interact with LasB and optimise the inhibitors across different parameters."
The compounds you developed do not kill the bacteria, but render them harmless. What is the advantage of this approach?
J. Haupenthal: "The fact that the bacteria's viability is not affected by our approach means, among other things, that the benign and beneficial bacteria, e.g. in the human intestine, are not endangered either. Another advantage is that we exert less selection pressure on the pathogens through such substances than antibiotics, since we are not trying to kill them. This should subsequently also reduce the likelihood of resistance developing. In addition, by administering our LasB inhibitors simultaneously with a standard-of-care antibiotic, the dose of the latter can be reduced."
In a mouse model you combined the developed compound with a classic antibiotic – why not just use the pathoblocker alone?
A. Kany: "The novel pathoblockers make the bacteria less harmful, but they do not kill them directly. Therefore, we combined the LasB inhibitor with the standard-of-care antibiotic levofloxacin and used a dose of this antibiotic that is not effective on its own. However, in combination with our pathoblocker, we observed an effect that confirms our working hypothesis that we can use pathoblockers to increase the efficacy of clinically used antibiotics."
What still needs to be done before this treatment option can be used in clinics?
A. Kany: "The dataset we currently have available, which is presented in this article, is very promising for further development, but of course further steps are needed to turn it into a drug. This includes further studies of efficacy in animal experiments, e.g. against different strains of P. aeruginosa including clinical isolates from patients. In addition, our data so far show that the compounds are well tolerated, do not target LasB-like enzymes from humans and are not toxic to human cells. While this is very promising, further, more complex safety features are needed on the way to clinical application, which we are now addressing step by step."
Dr Alwin Hartman conducted the interview.