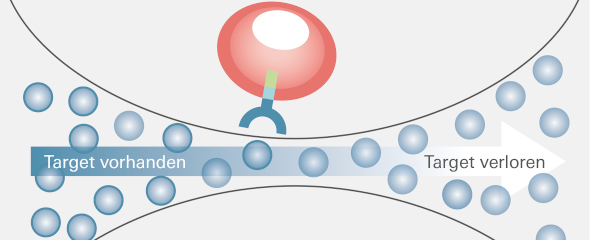
Multiple myeloma is a malignant cancer of the bone marrow. A great hope in the fight against the so far incurable disease rests on new immunotherapies, in particular on the treatment with CAR-T cells. T cells are white blood cells that serve the immune defense. In their natural state, they are mostly "blind" to tumor cells. However, they can be genetically modified to detect – as CAR-T cells – specific target antigens, i.e. proteins, on the tumor surface and subsequently destroy the cancer cells.
Severe relapse in myeloma patients
At the University Hospital of Würzburg (UKW), under the leadership of Prof Dr Hermann Einsele, Director of Medical Clinic II, an intensively pretreated myeloma patient with a poor prognosis was treated with CAR-T cells for the first time at the end of 2018 as part of a study. The treatment response was quite spectacular: Within a very short time, the bone marrow seemed to be completely cleared of tumor cells, and the myeloma indicators in the patient's blood also fell below the detection limit. However, this was only a temporary victory. After five months, there was a massive relapse: the bone marrow was again flooded with myeloma cells, and the patient died within a few weeks.
The target antigen was lost
In their search for the causes of this momentous course, researchers at UKW Medical Clinic II and the Würzburg-based Helmholtz Institute for RNA-based Infection Research (HIRI) uncovered a significant resistance mechanism, which they just published in the journal Nature Medicine. A key role in the observed resistance is played by BCMA, an antigen on the surface of myeloma cells used in CAR-T cell therapy. "We examined the patient's tumor cells at the time of relapse and found that BCMA, the target structure crucial for treatment success, was now completely absent," reports Dr Leo Rasche. The senior medical officer at UKW's Medical Clinic II is the initiator of the study.
Genetic variant benefits from selection pressure
To track down this initially unexplained loss, HIRI scientists analyzed the genome of thousands of the myeloma cells in question by single-cell RNA (ribonucleic acid) sequencing. "It turned out that the cancer cells that were newly formed during relapse lacked the gene segment that contains the code for BCMA," reports Dr Emmanuel Saliba, head of single-cell analysis at HIRI. The researchers believe that selection induced by CAR-T cell therapy is behind this. Dr Rasche explains, "In addition to the vast majority of myeloma cells with BCMA, there was probably an isolated genetic variant without BCMA even before treatment with CAR-T cells. While the cells with BCMA were successfully tracked down and eliminated, the remaining cells without BCMA had such a survival advantage that they subsequently grew rapidly."
Don't target CAR-T cell therapies to just one antigen
This finding may have implications for the design of future CAR-T cell therapies. "There is much to suggest that CAR-T cells should not be used to fire against just one specific antigen, but rather to address two or even three targets simultaneously," says Dr Rasche. Technically, this is, according to him, entirely possible: one could give a patient two CAR-T cell products at the same time, or use multispecific CAR-T cells that are equipped with multiple antigen receptors on their surface. "This would distribute the selection pressure, and the probability of tumor cells surviving would be significantly lower," the internist emphasizes.
Prof Dr Einsele adds: "The activity of CAR-T cells is impressive. It is therefore all the more important to understand the resistance mechanisms even better in order to further optimize this pioneering therapy. Single-cell RNA sequencing has proven to be an ideal screening tool for this." Prof Dr Jörg Vogel, Managing Director of the Würzburg-based Helmholtz Institute, says, "We are proud to be a global pioneer with our RNA technologies and to make our know-how available for clinical research here as well. At HIRI, it is an important concern for us to strengthen biomedical research at the science location Würzburg with our technologies."
Genetically predisposed risk group?
In addition to the Würzburg patient, Dr Rasche is aware of two other similar cases of resistance in myeloma patients in the USA and Canada. Nevertheless, it is not possible to estimate the frequency at this time. "At the moment, it is still open whether this mechanism occurs in all patients or only in a specific group," Rasche says. In this context, another observation of the Würzburg researchers is interesting: in about six percent of all myeloma patients, one of the otherwise duplicated BCMA-encoding genes is already missing even without CAR-T-cell therapy. This could be an indication of an increased risk for the described resistance mechanism.
Publication
Da Vià, M.C., Dietrich, O., Truger, M., Arampatzi, P., Duell, J., Heidemeier, A., Zhou, X., Danhof, S., Kraus, S., Chatterjee, M., Meggendorfer, M., Twardziok, S., Goebeler, M., Topp, M.S., Hudecek, M., Prommersberger, S., Hege, K., Kaiser, S., Fuhr, V., Weinhold, N., Rosenwald, A., Erhard, F., Haferlach, C., Einsele, H., Kortüm K. M., Saliba, A., Rasche, L. (2021) Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat Med