Antimicrobials through Chemoenzymatic Synthesis

Our research
Natural products (NPs) have historically played a crucial role in drug discovery due to their bioactivity-rich scaffolds, which serve as valuable templates for therapeutic agents. However, their use in drug development has dwindled, primarily because of the difficulties associated with isolating these compounds from natural sources or the limitations of conventional synthetic methods. By harnessing the unique reactivity and selectivity of enzymes from NP biosynthetic pathways combined with cutting-edge organic chemistry techniques, we aim to develop practical solutions to access those NPs and their analogs. Besides developing feasible synthetic routes for NPs active against Plasmodium falciparum, the causative pathogen of malaria, Mycobacterium tuberculosis and other microorganisms, we are focusing on the discovery of novel biocatalysts. By generating a biocatalyst library, we are aiming to fortify the traditional synthetic toolkit available for NP synthesis while providing deeper insights into underexplored enzyme chemistry.
Our research
Natural products (NPs) have historically played a crucial role in drug discovery due to their bioactivity-rich scaffolds, which serve as valuable templates for therapeutic agents. However, their use in drug development has dwindled, primarily because of the difficulties associated with isolating these compounds from natural sources or the limitations of conventional synthetic methods. By harnessing the unique reactivity and selectivity of enzymes from NP biosynthetic pathways combined with cutting-edge organic chemistry techniques, we aim to develop practical solutions to access those NPs and their analogs. Besides developing feasible synthetic routes for NPs active against Plasmodium falciparum, the causative pathogen of malaria, Mycobacterium tuberculosis and other microorganisms, we are focusing on the discovery of novel biocatalysts. By generating a biocatalyst library, we are aiming to fortify the traditional synthetic toolkit available for NP synthesis while providing deeper insights into underexplored enzyme chemistry.
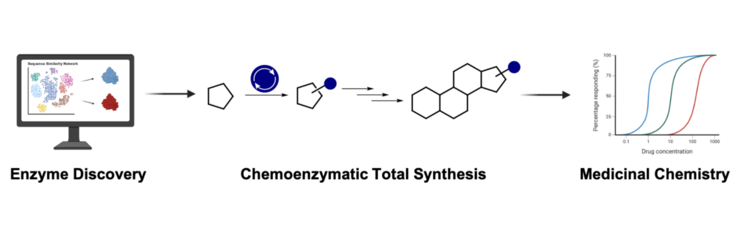
With the unmatched power of enzymatic reactions, we will fast-track natural product-based drug discovery
Having completed his PhD under the supervision of Prof. Uli Kazmaier at Saarland University, Alexander has honed skills in both classic total synthesis and medicinal chemistry-approaches to obtained cyclomarins with improved antimicrobial activities. During his short postdoctoral stay at Aalto University in Finland under the guidance of Prof. Dr. Jan Deska, he shifted from classical total synthesis to chemoenzymatic synthesis, focusing on the structurally unique angiopterlactone B. He successfully developed an efficient chemo-inspired biocatalytic total synthesis that integrated native enzymatic substrate conversion with artificial modules featuring abiotic transformations. After joining Prof. Dr. Anna Hirsch’s lab at the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) in mid-2019, he was involved in a variety of research projects centered on medicinal chemistry. With support of the DFG Walter Benjamin Program Fellowship, he transitioned to Prof. Dr. Ben Shen’s lab at Scripps Florida in early 2022, where he was a project leader, guiding an interdisciplinary team from three different labs. His research endeavors were centered on the development of anthraquinone-fused enediyne-based antibody-drug conjugates as promising anticancer agents. Upon returning to HIPS in mid-2024 on a DFG Walter Benjamin Return Fellowship, he was awarded the prestigious 21st Helmholtz Investigator Groups grant. This enabled him to establish his research group, focusing on natural product-inspired drug development at the interface of chemistry and biology.
Selected Publications
Alexander F. Kiefer*, Christian Schütz*, Colya N. Englisch, Dominik Kolling, Samira Speicher, Andreas M. Kany, Roya Shafiei, Noran A. Wadood, Ahmad Aljohmani, Niklas Wirschem, Ravindra P. Jumde, Andreas Klein, Asfandyar Sikandar, Yu-Mi Park, Gabriela Krasteva-Christ, Daniela Yildiz, Ahmed S. Abdelsamie, Katharina Rox, Jesko Köhnke, Rolf Müller, Markus Bischoff, Jörg Haupenthal and Anna K. H. Hirsch: "Dipeptidic Phosphonates: Potent Inhibitors of Pseudomonas aeruginosa Elastase B Showing Efficacy in a Murine Keratitis Model", Advanced Science 2024, 2411807. DOI: 10.1002/advs.202411807
Alexander F. Kiefer,* Yu-Chang Liu,* Rebecca Gummerer, Christina Jäger and Jan Deska: "An Artificial In Vitro Metabolism to Angiopterlactone B Inspired by Traditional Retrosynthesis", Angew. Chem. Int. Ed. 2023, 62, e202301178. DOI: 10.1002/anie.202301178
Andrew D. Steele,* Alexander F. Kiefer,* Dobeen Hwang,* Dong Yang, Christiana N. Teijaro, Ajeeth Adhikari, Christoph Rader, and Ben Shen: "Application of a Biocatalytic Strategy for the Preparation of Tiancimycin-based Antibody-Drug Conjugates Revealing Key Insights into Structure-Activity Relationships", Journal of Medicinal Chemistry 2023, 66, 1562–1573. DOI: 10.1021/acs.jmedchem.2c01771
Alexander F. Kiefer,* Spyridon Bousis,* Mostafa M. Hamed,* Eleonora Diamanti, Jörg Haupenthal and Anna K. H. Hirsch: "Structure-Guided Optimization of Small-Molecule Folate Uptake Inhibitors Targeting the Energy-Coupling Factor Transporters", Journal of Medicinal Chemistry2022, 65, 8869–8880. DOI: 10.1021/acs.jmedchem.1c02114
Alexander F. Kiefer, Chantal D. Bader, Jana Held, Anna Esser, Ran Rybniker, Martin Empting, Rolf Müller and Uli Kazmaier: "Synthesis of New Cyclomarin Derivatives and Their Biological Evaluation towards Mycobacterium Tuberculosis and Plasmodium Falciparum", Chem. Eur. J. 2019, 25, 8894–8902. DOI: 10.1002/chem.201901640
Publications
A complete list of publications can be found on the HIPS website.

![[Translate to English:] 3D-kontrastierte Oberflächenstruktur von LasB (beige) mit einem Inhibitor der 3. Generation (dunkelblau), der Zink (grau) bindet.](/fileadmin/_processed_/8/b/csm_LasB_Foto_b7734ae7a4.png)