Antiviral Antibody-Omics

Our Research
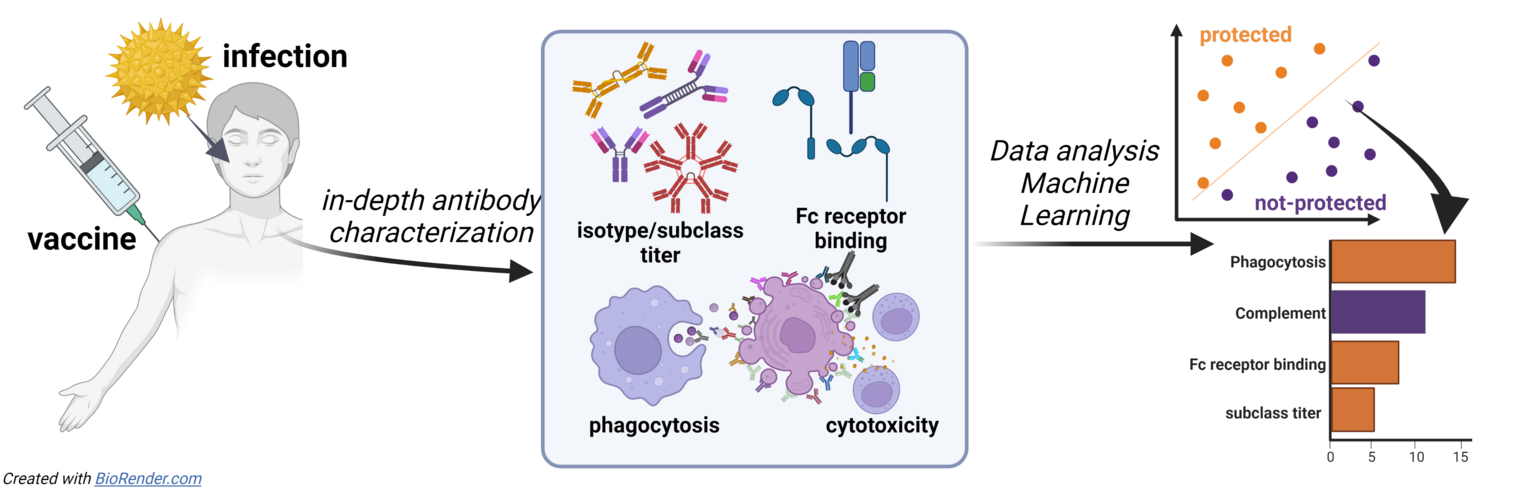
When an individual is infected with a pathogen, B cells start to secrete massive amounts of antibodies with a unique specificity that can recognise the pathogen. The variable Fab region of the antibody molecule mediates antigen-binding, which can prevent viral entry or neutralise pathogenic toxins.
The immune system's capacity to respond rapidly and effectively to the pathogen - in addition to binding and neutralisation - depends on the ability of the polyclonal antibody swarm to rapidly leverage and deploy opsonophagocytic clearance, drive cytotoxicity, and to stimulate the release of pro/anti-inflammatory mediators via Fc:Fc-receptor interaction on innate immune cells. These Fc mediated mechanism depend on a range of factors, including the expression patterns of activating and inhibitory Fc receptors on various immune effector cells, the selection of subclass and isotypes, as well as the accessibility of the Fc part and post-translational Fc modification (such as glycosylation) of the antigen-specific immune complexes.
Our translational research projects aim to broaden our knowledge of the role of antibody effector functions in infectious diseases, characterise the underlying mechanisms of Fc:Fc receptor interactions, and identify new approaches to improve antibody-based therapies in the future.
Recent data has shed light on the critical role of specific antibody effector profiles in combatting infectious diseases. However, certain features that are protective for certain pathogens may be ineffective or even detrimental in other contexts. Thus, to gain a comprehensive understanding of protective pathogen-specific responses, it is crucial to deeply analyse human cohorts to learn from natural examples of effective immune responses. Our research will utilise a systems immunology approach to thoroughly characterise protective antibody responses in different human cohorts of individuals who have either been naturally infected or vaccinated. By employing this approach, we can examine a range of biophysical properties, such as subclass and isotype distribution, Fc receptor binding, as well as innate effector functions like phagocytosis and cytotoxicity to identify specific characteristics of protective immunity.
Harnessing antibody Fc effector functions as therapeutic target
Monoclonal antibodies have emerged as extremely potent therapeutic molecules that have revolutionised the treatment of cancer and inflammatory diseases. With significant advancements in the field of infectious diseases, there has been an increasing push towards the development and approval of therapeutic anti-viral antibodies. Most if not all of these monoclonal antibodies have been screened and selected for their ability to neutralise and block cellular infection.
In our current research project, we aim to take this one step further and improve upon existing monoclonal antibody therapies by optimising their Fc effector functions. By leveraging the importance of Fc effector functions, we hope to develop novel investigational monoclonal antibody therapies that are even more effective in treating infectious diseases.
Our Research
When an individual is infected with a pathogen, B cells start to secrete massive amounts of antibodies with a unique specificity that can recognise the pathogen. The variable Fab region of the antibody molecule mediates antigen-binding, which can prevent viral entry or neutralise pathogenic toxins.
The immune system's capacity to respond rapidly and effectively to the pathogen - in addition to binding and neutralisation - depends on the ability of the polyclonal antibody swarm to rapidly leverage and deploy opsonophagocytic clearance, drive cytotoxicity, and to stimulate the release of pro/anti-inflammatory mediators via Fc:Fc-receptor interaction on innate immune cells. These Fc mediated mechanism depend on a range of factors, including the expression patterns of activating and inhibitory Fc receptors on various immune effector cells, the selection of subclass and isotypes, as well as the accessibility of the Fc part and post-translational Fc modification (such as glycosylation) of the antigen-specific immune complexes.
Our translational research projects aim to broaden our knowledge of the role of antibody effector functions in infectious diseases, characterise the underlying mechanisms of Fc:Fc receptor interactions, and identify new approaches to improve antibody-based therapies in the future.
Recent data has shed light on the critical role of specific antibody effector profiles in combatting infectious diseases. However, certain features that are protective for certain pathogens may be ineffective or even detrimental in other contexts. Thus, to gain a comprehensive understanding of protective pathogen-specific responses, it is crucial to deeply analyse human cohorts to learn from natural examples of effective immune responses. Our research will utilise a systems immunology approach to thoroughly characterise protective antibody responses in different human cohorts of individuals who have either been naturally infected or vaccinated. By employing this approach, we can examine a range of biophysical properties, such as subclass and isotype distribution, Fc receptor binding, as well as innate effector functions like phagocytosis and cytotoxicity to identify specific characteristics of protective immunity.
Harnessing antibody Fc effector functions as therapeutic target
Monoclonal antibodies have emerged as extremely potent therapeutic molecules that have revolutionised the treatment of cancer and inflammatory diseases. With significant advancements in the field of infectious diseases, there has been an increasing push towards the development and approval of therapeutic anti-viral antibodies. Most if not all of these monoclonal antibodies have been screened and selected for their ability to neutralise and block cellular infection.
In our current research project, we aim to take this one step further and improve upon existing monoclonal antibody therapies by optimising their Fc effector functions. By leveraging the importance of Fc effector functions, we hope to develop novel investigational monoclonal antibody therapies that are even more effective in treating infectious diseases.
Prof Dr Yannic Bartsch

Yannic Bartsch is the head of the Junior Research Group and W1 Professor for "Anti-viral Antibody-omics" at TWINCORE, a joint venture of the Helmholtz Centre for Infection Research (HZI) and the Hannover Medical School (MHH). His primary research focus is the analysis and understanding of the role of antibody-mediated effects in both health and disease, with a goal of utilising antibodies to mitigate disease. Yannic received his PhD from the University of Lübeck in Germany, where he investigated the protective effects of IgG Fc sialylation in murine models of autoimmune disease, as well as the mechanisms of adjuvant-dependent modification of IgG glycosylation upon vaccination in mice. As a postdoctoral researcher at the Ragon Institute of Mass General, MIT, and Harvard in Cambridge (USA), Yannic Bartsch studied the role of antibody Fc effector functions in viral infections, including HIV and SARS-CoV-2, under the mentorship of Prof Galit Alter and Prof Boris Julg. He made significant contributions to the field by addressing the role of Fc-mediated antibody effector functions in respiratory syncytial virus (RSV) infection and vaccination, and showing the relevance of Fc effector functions for protection from RSV infections. His research has resulted in numerous peer-reviewed first-author publications in high-impact journals.