
Our Research
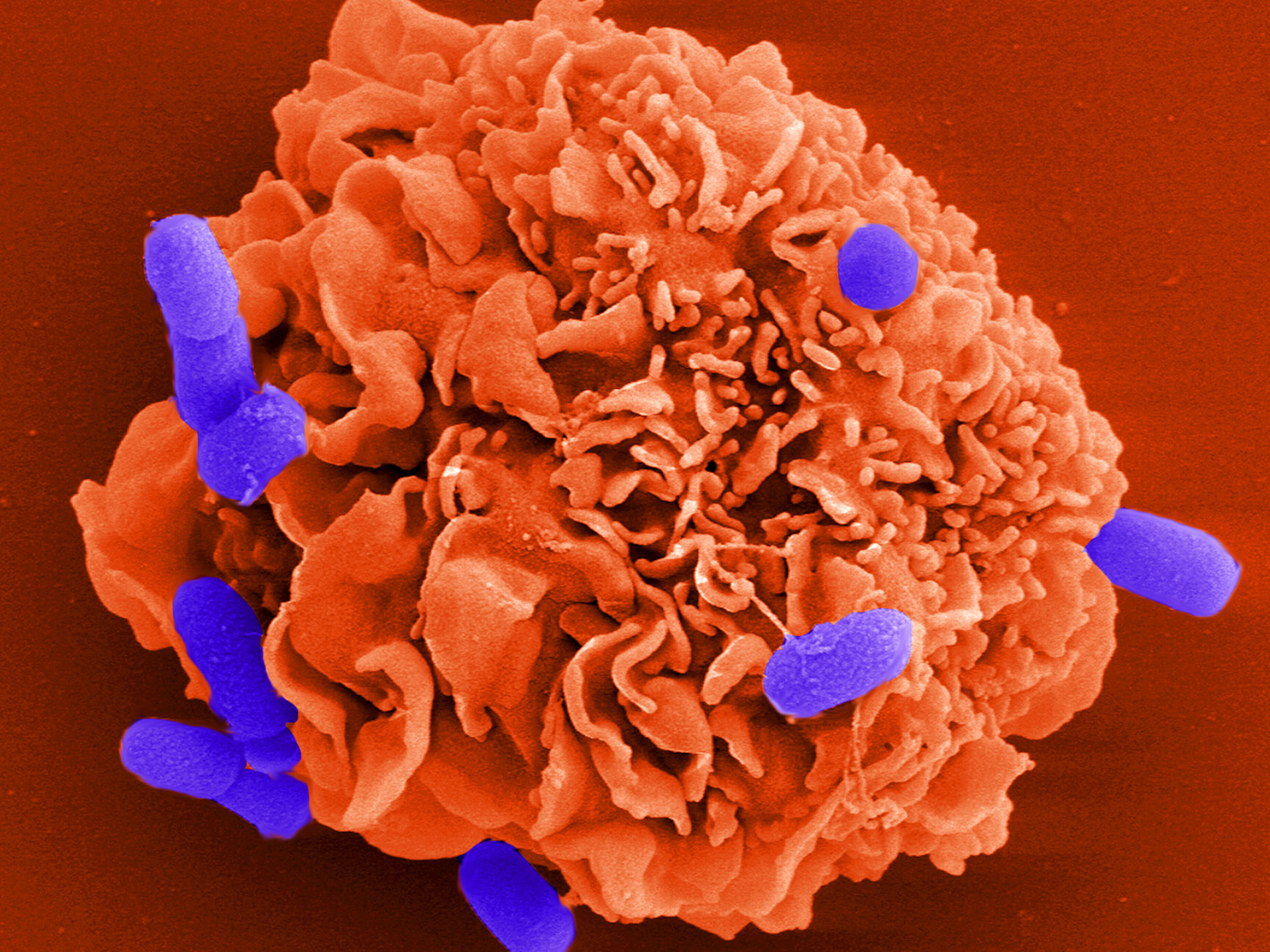
The HZI department Cell Biology looks at both the defense mechanisms of the host and the virulence mechanisms of the pathogens. Their core expertise is the combination of video-microscopy and protein biochemistry, applied to different models of cell movement in the context of bacterial infections. In the past, the department was able to observe regulatory mechanisms in cell movement and to find examples of how bacterial virulence factors influence them. Our research has shown that during the infection process bacterial pathogens are highly dependent on cooperating with host proteins – an insight which might lead to new strategies for fighting infections.
Our Research
The HZI department Cell Biology looks at both the defense mechanisms of the host and the virulence mechanisms of the pathogens. Their core expertise is the combination of video-microscopy and protein biochemistry, applied to different models of cell movement in the context of bacterial infections. In the past, the department was able to observe regulatory mechanisms in cell movement and to find examples of how bacterial virulence factors influence them. Our research has shown that during the infection process bacterial pathogens are highly dependent on cooperating with host proteins – an insight which might lead to new strategies for fighting infections.
Prof Dr Theresia Stradal
The interplay between pathogenic bacteria and cells of the host is tremendously complex and not well understood. A better understanding of this interface between molecules from both worlds, bacteria and host cells, bears the potential to find new ways to interrupt this often fatal interaction and might pave the way to novel anti-infective strategies.

Theresia Stradal studied Biology at the Paris Lodron University of Salzburg in Austria, where she also received her PhD from the Institute for Molecular Biology in 2000. She subsequently worked in the Cell Biology department at the Gesellschaft für Biotechnologische Forschung in Braunschweig (today’s Helmholtz Centre for Infection Research, HZI) as a postdoc for four years.
In 2005 she took over the group leader position until she was offered a W2 professorship by the Institute for Molecular Cell Biology at the University of Münster in 2009. In 2014, she returned to Braunschweig to accept a W3 professorship at the Institute for Zoology of the Technische Universität Braunschweig.
At the same time, Theresia Stradal became head of the department Cell Biology at the HZI. Her focus here is on host-pathogen-interactions as well as the defense mechanisms of the host and virulence mechanisms of the pathogens.
Team












Selected Publications
Stradal TE, Courtney KD, Rottner K, Hahne P, Small JV, Pendergast AM (2001) The Abl interactor proteins localize to sites of actin polymerization at the tips of lamellipodia and filopodia. Curr Biol 11: 891-895.
Steffen A, Rottner K, Ehinger J, Innocenti M, Scita G, Wehland J, Stradal TE (2004) Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J 23: 749-759.
Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp HJ, Milanesi F, Di Fiore PP, Ciliberto A, Stradal TE, Scita G (2006) Regulation of cell shape by Cdc42 is mediated by the synergic actin bundling activity of the Eps8:IRSp53 complex. Nat Cell Biol 8: 1337-1347.
Weiss SM, Ladwein M, Schmidt D, Ehinger J, Lommel S, Städing K, Beutling U, Disanza A, Frank R, Jänsch L, Scita G, Gunzer F, Rottner K, Stradal TE (2009) IRSp53 links Tir to EspFU/N-WASP-mediated actin assembly in EHEC pedestal formation. Cell Host Microbe 5: 244-258.
Hänisch J, Kölm R, Wozniczka M, Bumann D, Rottner K, Stradal TE (2011) Activation of a RhoA/myosin II-dependent but Arp2/3 complex-independent pathway facilitates Salmonella invasion. Cell Host Microbe 9: 273-285.
Publications
Review articles
Stradal TE, Costa SC. (2017) Type III Secreted Virulence Factors Manipulating Signaling to Actin Dynamics. Curr Top Microbiol Immunol. 399:175-199. doi: 10.1007/82_2016_35.
Rottner K, Stradal TE. (2016) How distinct Arp2/3 complex variants regulate actin filament assembly. Nat Cell Biol.;18(1):1-3.
Rottner, K., Stradal, T.E. (2011) Actin dynamics and turnover in cell motility. Curr. Opin. Cell. Biol. 23(5), 569-578.
Rottner, K., Stradal, T.E. (2009) Poxviruses taking a ride on actin: new users of known hardware. Cell Host & Microbe 6(6), 497-499.
Faix, J., Breitsprecher, D., Stradal, T.E., Rottner, K. (2009) Filopodia: Complex models for simple rods. Int. J. Biochem. Cell Biol. 41(8-9), 1656-1664.
Stradal, T.E., Scita, G. (2006) Protein complexes regulating cellular actin assembly. Curr. Op. Cell Biol. 18, 4-10.
Rottner, K., Stradal, T.E., Wehland, J. (2005) Bacteria – host cell interactions at the plasma membrane: stories on actin cytoskeleton subversion. Dev. Cell 9, 3-17.
Rottner, K., Lommel, S., Wehland, J., Stradal, T.E. (2004) Pathogen-induced actin filament rearrangement in infectious diseases. J. Pathol. 204, 396-406.
Stradal, T.E., Rottner, K., Disanza, A., Innocenti, M., Confalonieri, S., Scita, G. (2004). Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 14, 303-311.
Small, J.V., Stradal, T.E., Vignal, E., Rottner, K. (2002) The lamellipodium: where motility begins. Trends Cell Biol. 12, 112-120.
Stradal, T.E., Kranewitter, W., Winder, S.J., Gimona, M. (1998) CH domains revisited. FEBS Lett. 431, 134-137.
Book contributions
Steffen, A., Stradal, T.E., Rottner, K. (2016) Signalling pathways controlling cellular actin organization. The Actin Cytoskelton in “Handbook of Experimental Pharmacology” (ed. B. Jockusch) Springer Books (in press)
Stradal, T.E., Pusch, R., Kliche, S. (2006) Molecular regulation of cytoskeletal rearrangements during T cell signalling. In: Results and Problems in Cell Differentiation (RPCD); Volume: Cell Communication in Nervous and Immune System (eds. B. Schraven & E. Gundelfinger), Springer Verlag, Berlin, 43, pp219-244.
Rottner, K., Kaverina, I.N., Stradal, T.E. (2006) Cytoskeleton proteins. In “Cell Biology: A Laboratory Handbook”, 3rd Edition (ed. J.E. Celis), Academic Press, pp111-119.
Stradal, T.E., Lommel, S., Wehland, J., Rottner, K. (2005) Host-pathogen interactions and Cell Motility: Learning from Bacteria. In “Cell Migration in Development and Disease” (ed. D. Wedlich), WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, pp205-248.
Stradal, T.E., Sechi, A.S., Wehland, J., Rottner, K. (2003) The Cytoskeleton. In “Essential Cell Biology, Vol 1, ‘Cell Structure’: Practical Approach Series” (eds. M. Davey & M. Lord), Oxford University Press, pp365-389.
Other
Stradal, T.E., Backert, S. (2010) Host-pathogen interaction: How EHEC influence the actin cytoskeleton of the host cell. BioSpektrum 16 (6), 624-627.
Stradal, T.E. (2008) The regulation of cellular protrusions. Zellbiologie Aktuell 34(1), 17-22.
Stradal, T.E., Wehland, J. (2005) Aktindynamik und WASP/WAVE-Proteine. BioSpektrum 11(3), 283-286.
Ongoing Projects
Role of the WAVE complex in the immune system
Actin branching at the cell periphery depends on WAVE- and Arp2/3-complexes. Our group has a long history in WAVE-complex research and contributed significantly to the elucidation of its function. Over the years, we analyzed tissue-cultured cells suppressed in the expression of its subunits by RNA-interference or genetic deletion. To unambiguously clarify the role of WAVE-complex and thus lamellipodia in vivo, we decided to target WAVE-complex in the immune system, where motile cells fulfil defined functions while showing different types of motile behavior. Deletion of Hem1 results in the loss of WAVE-complex and consequently lamellipodium formation in all immune cells. Strikingly, Hem1 deletion eliminates chemotaxis. In complex 3D-environments, lamellipodia mediate exploration of the environment. Consequently, Hem1-null cells can no longer make directional decisions, persistently crawl in one direction and thus likely become trapped in narrow pores. These trapped yet activated innate immune cells then cause local sterile inflammation. Dendritic cells derived from these mice show abberant migratory behavior and fail to perform interstitial migration and chemotaxis (Leithner et al., 2016, Thiam et al., 2016).
Funding Agency:
DFG - German Research Foundation
Relevant publications concerning this project
I. Leithner A, Eichner A, Müller J, Reversat A, Brown M, Schwarz J, Merrin J, de Gorter DJ, Schur F, Bayerl J, de Vries I, Wieser S, Hauschild R, Lai FP, Moser M, Kerjaschki D, Rottner K, Small JV, Stradal TE, Sixt M (2017) Diversified actin protrusions promote environmental exploration but are dispensable for locomotion of leukocytes. Nat Cell Biol 18(11): 1253-59.
II. Thiam HR, Vargas P, Carpi N, Crespo CL, Raab M, Terriac E, King MC, Jacobelli J, Alberts AS, Stradal T, Lennon-Dumenil AM, Piel M (2016) Perinuclear Arp2/3-driven actin polymerization enables nuclear deformation to facilitate cell migration through complex environments. Nat Commun 7: 10997.
III. Kage F, Winterhoff M, Dimchev V, Mueller J, Thalheim T, Freise A, Brühmann S, Kollasser J, Block J, Dimchev G, Geyer M, Schnittler HJ, Brakebusch C, Stradal TE, Carlier MF, Sixt M, Käs J, Faix J, Rottner K (2017) FMNL formins boost lamellipodial force generation. Nat Commun 8:14832.
IV. Rottner K, Stradal TE (2016) How distinct Arp2/3 complex variants regulate actin filament assembly. Nat Cell Biol 18(1): 1-3.
V. Litschko C, Linkner J, Brühmann S, Stradal TEB, Reinl T, Jänsch L, Rottner K, Faix J (2017) Differential functions of WAVE regulatory complex subunits in the regulation of actin-driven processes. Eur J Cell Biol 96(8): 715-27.
VI. Steffen A, Ladwein M, Dimchev GA, Hein A, Schwenkmezger L, Arens S, Ladwein KI, Margit Holleboom J, Schur F, Victor Small J, Schwarz J, Gerhard R, Faix J, Stradal TE, Brakebusch C, Rottner K (2013) Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J Cell Sci 126(Pt 20): 4572-88.
VII. Tahirovic S, Hellal F, Neukirchen D, Hindges R, Garvalov BK, Flynn KC, Stradal TE, Chrostek-Grashoff A, Brakebusch C, Bradke F (2010) Rac1 regulates neuronal polarization through the WAVE complex. J Neurosci 30(20): 6930-43.
VIII. Derivery E, Fink J, Martin D, Houdusse A, Piel M, Stradal TE, Louvard D, Gautreau A (2008) Free Brick1 is a trimeric precursor in the assembly of a functional wave complex. PLoS ONE 3(6): e2462.
IX. Steffen A, Faix J, Resch GP, Linkner J, Wehland J, Small JV, Rottner K, Stradal TE (2006) Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol Biol Cell 17(6): 2581-91.
X. Stradal TE, Scita G (2006) Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol 18(1): 4-10.
XI. Innocenti M, Gerboth S, Rottner K, Lai FP, Hertzog M, Stradal TE, Frittoli E, Didry D, Polo S, Disanza A, Benesch S, Di Fiore PP, Carlier MF, Scita G (2005) Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol 7(10): 969-76.
XII. Innocenti M, Zucconi A, Disanza A, Frittoli E, Areces LB, Steffen A, Stradal TE, Di Fiore PP, Carlier MF, Scita G (2004) Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat Cell Biol 6(4): 319-27.
XIII. Steffen A, Rottner K, Ehinger J, Innocenti M, Scita G, Wehland J, Stradal TE (2004) Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J 23(4): 749-59.
XIV. Kaverina I, Stradal TE, Gimona M (2003) Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J Cell Sci 116(Pt 24): 4915-24.
XV. Small JV, Stradal T, Vignal E, Rottner K (2002) The lamellipodium: where motility begins. Trends Cell Biol 12(3): 112-20.
XVI. Stradal T, Courtney KD, Rottner K, Hahne P, Small JV, Pendergast AM (2001) The Abl interactor proteins localize to sites of actin polymerization at the tips of lamellipodia and filopodia. Curr Biol 11(11): 891-5.
Technology Offers
The following technologies have been developed and patented by the department "Cell Biology":
Efficient Generation of Murine Brain Organoids from Adult Neural Stem Cells