Central Facility for Microscopy


Our Expertise
The facility provides access to several fluorescence microscopes (FM), confocal microscopes (CLSM), two transmission electron microscopes (TEM), and a high-resolution field emission scanning electron microscope (FESEM) in addition to the peripheral preparation equipment.
The ZEIM team cooperates with the customer to establish a tailored preparation protocol and imaging technique/s to select the appropriate methodology for each research project. ZEIM has a portfolio of different methods available for preparing biological or material samples for light and electron microscopy.
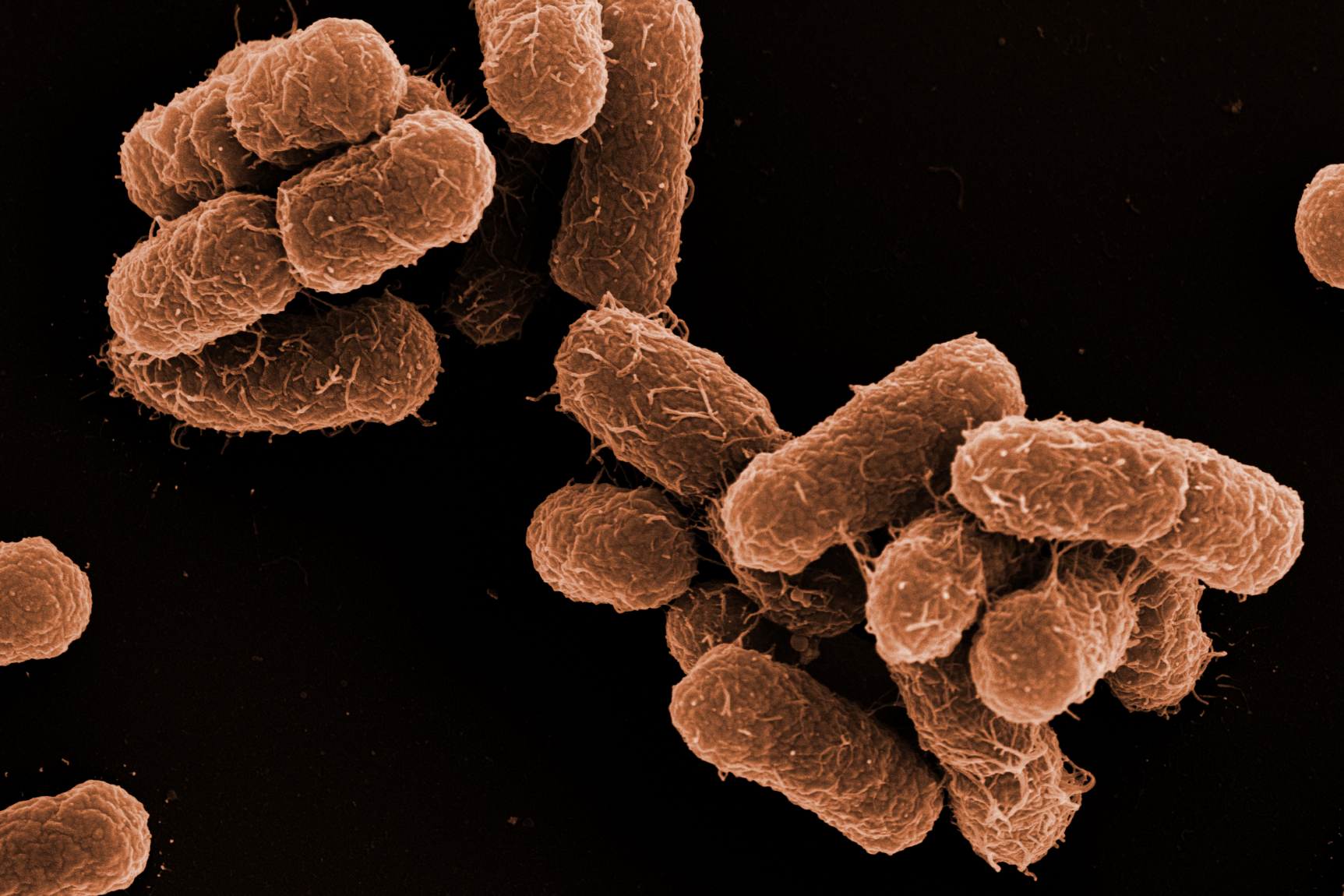
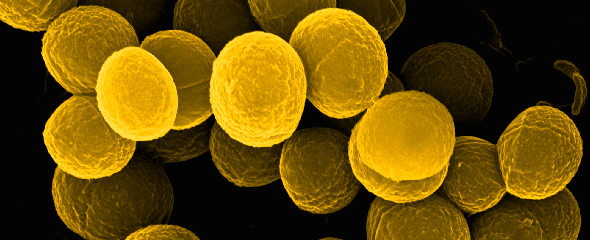
The outstanding expertise in the preparation of biological samples for high quality imaging is a key strength of our EM service. The morphological analysis from new isolated microorganisms and bacteriophages is another. Furthermore, ZEIM offers to perform infection experiments with pathogenic bacteria on cell lines with subsequent preparation of samples, image acquisition and followed by analysis for publications.
We provide support and assistance for imaging with a fluorescence or confocal microscope and a tailored training to the customers’ needs. Besides classical fluorescence labeling studies, double immuno labeling for discriminating between surface adhesion and intracellular uptake of bacterial pathogens are well-established methods in our facility, which can be provided on demand.
Transmission Electron Microscopy (TEM)
Besides negative-staining for bacteria, bacteriophages or vesicles, representing a rather fast method in EM, a portfolio of different embedding schemes are available. This includes low temperature embedding and high pressure freezing with freeze substitution and subsequent analysis of samples in ultrathin sections via TEM. ZEIM has also experience in performing immuno cytochemical stainings for detecting pathogenicity factors with the help of antibodies and gold nanoparticles in eukaryotic cells or bacteria.
EQUIPMENT:
- Transmission electron microscope Zeiss Libra120 Plus
- Transmission electron microscope Zeiss TEM910
- Ultramicrotome Leica UC7 with cryo chamber FC7
- Ultramicrotome Reichert Ultracut S
- High Pressure Freezer Leica EM-Pact 1
- Freeze substitution Leica EM AFS
- Coating Unit Bal-Tec MED 020
Field Emission Scanning Electron Microscopy (FESEM)
For high-resolution FESEM studies standard protocols have been established which should result in a first satisfying preparation of samples to be investigated. This way infection processes and surface structures can be precisely visualized. However, new preparation methods will be customized and established if needed for special experiments. Similar to TEM, FESEM can be coupled with immuno cytometry to select your targets of interest.
EQUIPMENT:
- Field emission scanning electron microscope Zeiss Merlin with Atlas, STEM Detector, EsB-Detector for material contrast, Shuttle&Find for CLEM (correlative light- and electron microscopy), and Oxford EDX Aztec with X-Max Detector
- Critical-Point Drying Leica EM CPD300
- Sputter Coater Bal-Tec SCD 500
Fluorescence (FM) and confocal laser-scanning microscopy (CLSM)
FM/CLSM offer the possibilities to visualize selectively stained compartments of your cells. Thereby staining is achieved by various means including fluorescent dyes, fluorescent proteins or fluorescently labeled antibodies. The big advantage towards EM: You can visualize both live and fixed specimen. In case of three-dimensional structures CLSM is the microscope of choice since it allows acquiring optical sections, which can be reconstructed to 3D volumes afterwards with the commercial image analysis software Imaris, which is available in ZEIM.
EQUIPMENT:
- Zeiss Axio Imager A2
- Zeiss Axio Imager A1
- Zeiss Axio Observer Z1 with apotome
- Zeiss Imager Z2
- Leica SP5 upright confocal laser-scanning microscope
- Leica SP8 inverse confocal laser-scanning microscope
- Image analysis software: Imaris version 9.3. (Oxford Instruments)
Our Expertise
The facility provides access to several fluorescence microscopes (FM), confocal microscopes (CLSM), two transmission electron microscopes (TEM), and a high-resolution field emission scanning electron microscope (FESEM) in addition to the peripheral preparation equipment.
The ZEIM team cooperates with the customer to establish a tailored preparation protocol and imaging technique/s to select the appropriate methodology for each research project. ZEIM has a portfolio of different methods available for preparing biological or material samples for light and electron microscopy.
The outstanding expertise in the preparation of biological samples for high quality imaging is a key strength of our EM service. The morphological analysis from new isolated microorganisms and bacteriophages is another. Furthermore, ZEIM offers to perform infection experiments with pathogenic bacteria on cell lines with subsequent preparation of samples, image acquisition and followed by analysis for publications.
We provide support and assistance for imaging with a fluorescence or confocal microscope and a tailored training to the customers’ needs. Besides classical fluorescence labeling studies, double immuno labeling for discriminating between surface adhesion and intracellular uptake of bacterial pathogens are well-established methods in our facility, which can be provided on demand.
Transmission Electron Microscopy (TEM)
Besides negative-staining for bacteria, bacteriophages or vesicles, representing a rather fast method in EM, a portfolio of different embedding schemes are available. This includes low temperature embedding and high pressure freezing with freeze substitution and subsequent analysis of samples in ultrathin sections via TEM. ZEIM has also experience in performing immuno cytochemical stainings for detecting pathogenicity factors with the help of antibodies and gold nanoparticles in eukaryotic cells or bacteria.
EQUIPMENT:
- Transmission electron microscope Zeiss Libra120 Plus
- Transmission electron microscope Zeiss TEM910
- Ultramicrotome Leica UC7 with cryo chamber FC7
- Ultramicrotome Reichert Ultracut S
- High Pressure Freezer Leica EM-Pact 1
- Freeze substitution Leica EM AFS
- Coating Unit Bal-Tec MED 020
Field Emission Scanning Electron Microscopy (FESEM)
For high-resolution FESEM studies standard protocols have been established which should result in a first satisfying preparation of samples to be investigated. This way infection processes and surface structures can be precisely visualized. However, new preparation methods will be customized and established if needed for special experiments. Similar to TEM, FESEM can be coupled with immuno cytometry to select your targets of interest.
EQUIPMENT:
- Field emission scanning electron microscope Zeiss Merlin with Atlas, STEM Detector, EsB-Detector for material contrast, Shuttle&Find for CLEM (correlative light- and electron microscopy), and Oxford EDX Aztec with X-Max Detector
- Critical-Point Drying Leica EM CPD300
- Sputter Coater Bal-Tec SCD 500
Fluorescence (FM) and confocal laser-scanning microscopy (CLSM)
FM/CLSM offer the possibilities to visualize selectively stained compartments of your cells. Thereby staining is achieved by various means including fluorescent dyes, fluorescent proteins or fluorescently labeled antibodies. The big advantage towards EM: You can visualize both live and fixed specimen. In case of three-dimensional structures CLSM is the microscope of choice since it allows acquiring optical sections, which can be reconstructed to 3D volumes afterwards with the commercial image analysis software Imaris, which is available in ZEIM.
EQUIPMENT:
- Zeiss Axio Imager A2
- Zeiss Axio Imager A1
- Zeiss Axio Observer Z1 with apotome
- Zeiss Imager Z2
- Leica SP5 upright confocal laser-scanning microscope
- Leica SP8 inverse confocal laser-scanning microscope
- Image analysis software: Imaris version 9.3. (Oxford Instruments)
Our Research
In addition to the actual service work, we are actively involved in scientific collaborations. We use special imaging techniques and corresponding analyses to address scientific questions accordingly and support our partners with strong microbiological and cell biological expertise. The main focus of our research is the visualization of drug kinetics of antimicrobial compounds within bacterial communities - so-called biofilms - using various microscopy methods, with a particular focus on the two clinically relevant model organisms Pseudomonas aeruginosa and Staphylococcus aureus. However, there are also ongoing projects with outstanding cooperation partners dealing with streptococci bacteria.




Selected Publications
Osbelt L, Almási ÉDH, Wende M, Kienesberger S, Voltz A, Lesker TR, Muthukumarasamy U, Knischewski N, Nordmann E, Bielecka AA, Giralt-Zúñiga M, Kaganovitch E, Kühne C, Baier C, Pietsch M, Müsken M, Greweling-Pils MC, Breinbauer R, Flieger A, Schlüter D, Müller R, Erhardt M, Zechner EL, Strowig T. Klebsiella oxytoca inhibits Salmonella infection through multiple microbiotacontext-dependent mechanisms. Nat Microbiol. 2024 Jul;9(7):1792-1811. doi: 10.1038/s41564-024-01710-0
Bublitz A, Brauer M, Wagner S, Hofer W, Müsken M, Deschner F, Lesker TR, Neumann-Schaal M, Paul LS, Nübel U, Bartel J, Kany AM, Zühlke D, Bernecker S, Jansen R, Sievers S, Riedel K, Herrmann J, Müller R, Fuchs TM, Strowig T. The natural product chlorotonil A preserves colonization resistance and prevents relapsing Clostridioides difficile infection. Cell Host Microbe. 2023 May 10;31(5):734-750.e8. doi: 10.1016/j.chom.2023.04.003.
Thöming JG, Tomasch J, Preusse M, Koska M, Grahl N, Pohl S, Willger SD, Kaever V, Müsken M, Häussler S. Parallel evolutionary paths to produce more than one Pseudomonas aeruginosa biofilm phenotype. NPJ Biofilms Microbiomes. 2020 Jan 10;6:2. doi: 10.1038/s41522-019-0113-6.
Müsken M, Pawar V, Schwebs T, Bähre H, Felgner S, Weiss S, Häussler S. Breaking the vicious cycle of antibiotic killing and regrowth of biofilm-residing Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2018 Nov 26;62(12):e01635-18. doi: 10.1128/AAC.01635-18
Müsken M, Di Fiore S, Römling U, Häussler S. A 96-well-plate-based optical method for the quantitative and qualitative evaluation of Pseudomonas aeruginosa biofilm formation and its application to susceptibility testing. Nat Protoc. 2010 Aug;5(8):1460-9. doi:10.1038/nprot.2010.110