Chemical Biology of Carbohydrates

Our Research
Treatment of chronic infections: disrupting lectin-mediated biofilms
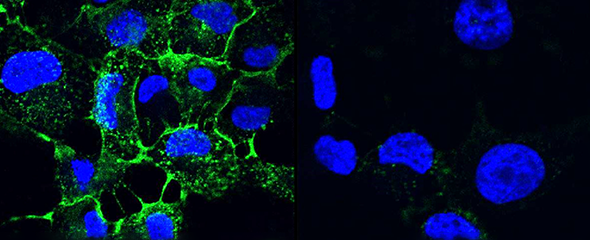
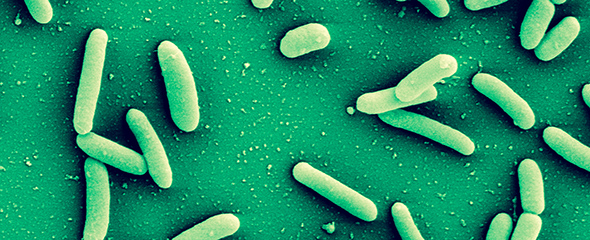
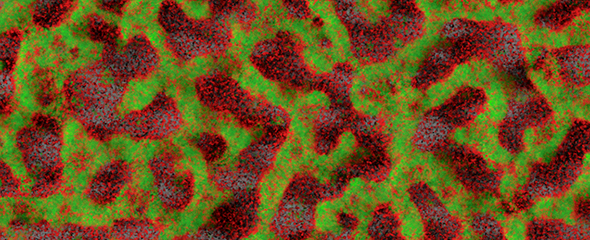
Many human pathogens can establish chronic infections with the help of a biofilm mode of life. As a protective shield, the matrix of the biofilm renders antibiotics ineffective and secures survival of the embedded pathogen. Novel ways for treatment address disintegration of such biofilms and thus restore activity of antibiotics. Frequently, the architecture of biofilms is maintained by carbohydrates and so-called lectins, recognizing and cross-linking carbohydrate motifs of the glycocalyx, both on human cells and pathogens. The inhibition of such structural components leads to the disruption of a biofilm and thereby allows treatment of the infection. Pseudomonas aeruginosa is an important pathogen in hospital-acquired infections and for cystic fibrosis patients. This Gram-negative bacterium can establish chronic infections in various tissues through assembly into protective biofilms. One focus of our research is two P. aeruginosa lectins, which are crucial elements of the biofilm architecture.
Many human pathogens can establish chronic infections with the help of a biofilm mode of life. As a protective shield, the matrix of the biofilm renders antibiotics ineffective and secures survival of the embedded pathogen. Novel ways for treatment address disintegration of such biofilms and thus restore activity of antibiotics. Frequently, the architecture of biofilms is maintained by carbohydrates and so-called lectins, recognizing and cross-linking carbohydrate motifs of the glycocalyx, both on human cells and pathogens. The inhibition of such structural components leads to the disruption of a biofilm and thereby allows treatment of the infection. Pseudomonas aeruginosa is an important pathogen in hospital-acquired infections and for cystic fibrosis patients. This Gram-negative bacterium can establish chronic infections in various tissues through assembly into protective biofilms. One focus of our research is two P. aeruginosa lectins, which are crucial elements of the biofilm architecture.
The group of Prof. Alexander Titz aims at the development of antibacterial drugs using a combination of medicinal chemistry, biochemistry and microbiological methods. Recently, a competitive binding assay was developed for the in vitro evaluation of inhibitors of the Pseudomonas lectins. In collaboration with other groups at HIPS and the HZI, potent molecules obtained by the group are then further evaluated in biofilm and infection models. Such compounds may ultimately lead to successful treatment of chronic infections without evoking resistances among the pathogens.
Prof. Titz heads the Chair of Organic and Pharmaceutical Chemistry at Saarland University as well as the Chemical Biology of Carbohydrates group at the HZI/HIPS. The information about projects, staff, and publications on this website covers both groups.
Our Research
Treatment of chronic infections: disrupting lectin-mediated biofilms
Many human pathogens can establish chronic infections with the help of a biofilm mode of life. As a protective shield, the matrix of the biofilm renders antibiotics ineffective and secures survival of the embedded pathogen. Novel ways for treatment address disintegration of such biofilms and thus restore activity of antibiotics. Frequently, the architecture of biofilms is maintained by carbohydrates and so-called lectins, recognizing and cross-linking carbohydrate motifs of the glycocalyx, both on human cells and pathogens. The inhibition of such structural components leads to the disruption of a biofilm and thereby allows treatment of the infection. Pseudomonas aeruginosa is an important pathogen in hospital-acquired infections and for cystic fibrosis patients. This Gram-negative bacterium can establish chronic infections in various tissues through assembly into protective biofilms. One focus of our research is two P. aeruginosa lectins, which are crucial elements of the biofilm architecture.
Many human pathogens can establish chronic infections with the help of a biofilm mode of life. As a protective shield, the matrix of the biofilm renders antibiotics ineffective and secures survival of the embedded pathogen. Novel ways for treatment address disintegration of such biofilms and thus restore activity of antibiotics. Frequently, the architecture of biofilms is maintained by carbohydrates and so-called lectins, recognizing and cross-linking carbohydrate motifs of the glycocalyx, both on human cells and pathogens. The inhibition of such structural components leads to the disruption of a biofilm and thereby allows treatment of the infection. Pseudomonas aeruginosa is an important pathogen in hospital-acquired infections and for cystic fibrosis patients. This Gram-negative bacterium can establish chronic infections in various tissues through assembly into protective biofilms. One focus of our research is two P. aeruginosa lectins, which are crucial elements of the biofilm architecture.
The group of Prof. Alexander Titz aims at the development of antibacterial drugs using a combination of medicinal chemistry, biochemistry and microbiological methods. Recently, a competitive binding assay was developed for the in vitro evaluation of inhibitors of the Pseudomonas lectins. In collaboration with other groups at HIPS and the HZI, potent molecules obtained by the group are then further evaluated in biofilm and infection models. Such compounds may ultimately lead to successful treatment of chronic infections without evoking resistances among the pathogens.
Prof. Titz heads the Chair of Organic and Pharmaceutical Chemistry at Saarland University as well as the Chemical Biology of Carbohydrates group at the HZI/HIPS. The information about projects, staff, and publications on this website covers both groups.
Prof Dr Alexander Titz
We are looking for molecules that selectively block the individual biofilm components of resistant chronic infections to restore therapeutic interventions with antibiotics.

Alexander Titz studied chemistry at the Technical University Darmstadt and the University of Bordeaux I. His diploma thesis was prepared at Novartis Pharma AG in the Protein Structure Unit.
After receiving his doctorate from the University of Basel in 2008, covering the medicinal chemistry of carbohydrate-protein interactions, he conducted post-doctoral research at the ETH Zürich (Swiss Federal Institute of Technology) at the Institute of Microbiology and Immunology. In 2010 he was awarded the Klaus-Grohe Prize for medicinal chemistry of the GDCh. Alexander Titz then moved to the Zukunftskolleg of the University of Constance as a group leader and focused on inhibitors of bacterial biofilm formation.
Since 2013, he is group leader of the workgroup Chemical Biology of Carbohydrates at Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), an outpost of the HZI.
Since 2017, the research in the Titz lab is funded by an ERC starting grant. 2018 Alexander Titz was awarded the Innovation Award in Medical/Pharmaceutical Chemistry by the Gesellschaft Deutscher Chemiker and the Deutsche Pharmazeutische Gesellschaft.
Furthermore, in 2018 Alexander Titz received the Prize for a Young Medicinal Chemist in Academia of the European Federation of Medicinal Chemistry (EFMC) for the research of his team. In 2019, he obtained a call for the professorship for Organic Chemistry at the University of Osnabrück. Since 2020, Alexander Titz holds a professorship for Organic and Pharmaceutical Chemistry at Saarland University.
Selected Publications
Zahorska, E.; Rosato, F.; Stober, K.; Kuhaudomlarp, S.; Meiers, J.; Hauck, D.; Reith, D.; Gillon, E.; Rox, K.; Imberty, A.; Römer, W.*, Titz, A.* Neutralizing the impact of the virulence factor LecA from Pseudomonas aeruginosa on human cells with new glycomimetic inhibitors, Angew. Chem. Int. Ed. Engl. 2023, 62(7), e202215535, DOI: 10.1002/anie.202215535
Leusmann, S.; Menova, P.; Shanin, E.; Titz, A.*, Rademacher, C.* Glycomimetics for the inhibition and modulation of lectins, Chem. Soc. Rev. 2023, 52, 3663-3740, DOI: 110.1039/D2CS00954D
Kuhaudomlarp, S.; Siebs, E.; Shanina, E.; Topin, J.; Joachim, I.; da Silva Figueiredo Celestino Gomes, P.; Varrot, A.; Rognan, D.; Rademacher, C.; Imberty, A.*, Titz, A.* Non-Carbohydrate Glycomimetics as Inhibitors of Calcium(II)-binding Lectins. Angew. Chem. Int. Ed. Engl. 2021, 60(15), 8104-8114, DOI: 10.1002/anie.202013217
Sommer, R.; Wagner, S.; Rox, K.; Varrot, A.; Hauck, D.; Wamhoff, E.-C.; Schreiber, J.; Ryckmans, T.; Brunner, T.; Rademacher, C.; Hartmann, R. W.; Brönstrup, M.; Imberty, A.; Titz, A.* Glycomimetic, orally bioavailable LecB inhibitors block biofilm formation of Pseudomonas aeruginosa. J. Am. Chem. Soc. 2018, 140(7), 2537-2545, DOI: 10.1021/jacs.7b11133
Wagner, S.; Hauck, D.; Hoffmann, M.; Sommer, R.; Joachim, I.; Müller, R.; Imberty, A.; Varrot, A.; Titz, A.* Covalent lectin inhibition and its application in bacterial biofilm imaging. Angew. Chem. Int. Ed. Engl. 2017, 56, 16559-16564. DOI: 10.1002/anie.201709368
A complete list of publications can be found on the HIPS website
Technology Offers
The following technologies have been developed and patented by the group Chemical Biology of Carbohydrates:
Bivalent LecA Inhibitors Targeting Biofilm Formation of Pseudomonas aeruginosa
Novel inhibitors of Pseudomonas aeruginosa LecB
Projects
SIALOMIMETIC VIRAL ENTRY INHIBITORS - A ROUTE TOWARDS THE FIRST TREATMENTS FOR NEGLECTED HUMAN AND EMERGING ZOONOTIC VIRUSES (ALEXANDER TITZ & DOMINIQUE SCHOLS)
Zoonotic viruses can rapidly emerge from animals and transmit to humans as has been well documented for various avian/porcine influenza and bat corona viruses. It has now been observed repeatedly that these animal-to-human transmissions can result in a pandemic causing millions of deaths and a massive economic burden for humanity. All those pandemic viruses and numerous other classes of human viruses exploit animal and human sialic acid receptors for transmission and infection. Therefore, viral entry inhibitors interfering with this process are of broad interest. In this early discovery project, the development of novel sialomimetic therapeutics will be established. After initial validation using known viruses with known and distinct sialic acid binding specificity, the platform will then be applied onto viruses with reported but unexplored sialic acid binding, i.e. Parainfluenza, Zika and BK Polyoma Viruses, all of which currently lack drugs or vaccines. This pipeline will therefore yield sialomimetic entry inhibitors for the treatment of viral infections with clinical need, and importantly, the established workflow will serve as an important platform in the scope of Pandemic Preparedness.
CARBOHYDRATE CONJUGATES AS TROJAN HORSE TO IMPROVE ANTIBIOTIC IMPORT
The increase of antimicrobial resistance is a major threat for individual and public health. Consequently, new weapons to fight the emerging multidrug-resistant bacteria are quickly required. The ESKAPE pathogens are at the frontline of these problematic life-threatening drug resistant nosocomial pathogens. In this project, we aim to overcome antimicrobial resistance by hijacking energy-driven carbohydrate uptake systems (PTS and ABC transporters) to actively pump carbohydrate-antibiotic conjugates into bacteria which will be liberated by cellular glycosidases. These transporters are able to accumulate mM intracellular concentrations of their substrates. Exploring if they will boost intracellular concentrations of the carbohydrate-antibiotic conjugates as well is the main objective of the CO-TEAM project. Our preliminary work shows that the expression of the transporters of these carbohydrates is significantly induced under infection conditions. The project is divided into 4 tasks. 4 different oligosaccharide conjugates carrying 6 different antibiotics from 4 different classes will be chemically synthesized. These conjugates will then be studied for antimicrobial efficacy and resistance development against a panel of strains with a focus on the most urgent ESKAPE pathogens. In addition, mechanistic analyses to identify the transporters importing the conjugates will be conducted in 2 Gram-positive and 2 Gram-negative pathogens. Finally, we will evaluate the most promising conjugates in an insect and mouse model. Boosting intracellular antibiotic concentrations through active import is expected to overcome antimicrobial resistance mechanisms, both, target modifications lowering the antibiotic's efficacy, also permeability affecting resistance, the major problem in Gram-negative bacteria.
SWEETBULLETS (JOSCHA MEIERS, OLGA METELKINA, VARVARA VERKHOVA AND EVA ZAHORSKA)
Bacterial infections are now a global threat demanding novel treatments due to the appearance of resistances against antibiotics at a high pace. The ESKAPE pathogens are those with highest importance in the EU and chronic infections due to biofilm formation are a particular task. Noninvasive pathogen-specific imaging of the infected tissue is not clinically available. Its successful implementation will enable the choice of appropriate therapy and boost efficacy. Furthermore,
Gram-negative bacteria have a highly protective cellular envelope as an important resistance mechanism for drugs acting intracellularly, resulting in an alarmingly empty drug-pipeline.
To overcome this gap, I will establish Lectin-directed Theranostics targeting pathogens via their extracellular carbohydrate-binding proteins at the site of infection for specific imaging and treatment. This will be implemented for the highly resistant ESKAPE pathogen Pseudomonas aeruginosa through 3 different work packages.
WP1 Sweet Imaging: Design & conjugation of lectin-directed ligands to imaging probes, Optimization of ligand/linker, in vivo proof-of-concept imaging study.
WP2 Sweet Targeting: Delivery of antibiotics to the infection through covalent linking of lectindirecting groups. Employing different antibiotics, assessment of bactericidal potency and targeting efficiency. Manufacturing of nano-carriers with surface exposed lectin-directed ligands, noncovalent charging with antibiotics. In vitro and in vivo targeting.
WP3 Sweet SMART Targeting: Conjugates as SMART drugs: specific release of anti-biofilm lectin inhibitor and drug cargo upon contact with pathogen, development of linkers cleavable by pathogenic enzymes.
SWEETBULLETS will establish fundamentally novel lectin-directed theranostics to fight these deleterious infections and provide relief to nosocomially infected and cystic fibrosis patients. It is rapidly extendable towards other ESKAPE pathogens, e.g. Klebsiella spp..
DEVELOPMENT OF NON-CARBOHYDRATE GLYCOMIMETICS (EIKE SIEBS)
Kohlenhydrat-Protein-Wechselwirkungen sind häufig an Schlüsselstellen von biologischen Prozessen beteiligt, wie der Befruchtung von Eizellen, der Rekrutierung von Immunzellen in Gewebe, aber auch bei Infektionsprozessen, bei Entzündungen, bei der Tumormetastasierung und anderen pathophysiologischen Konditionen. Pathogene Mikroorganismen, z. B. Viren, Bakterien, Pilze oder Parasiten, haben verschiedene Strategien entwickelt, um Zuckerstrukturen auf menschlichem Gewebe spezifisch zu erkennen oder diese für den Zelleintritt zu nutzen. Beispiele für Proteine in solchen Erkennungsprozessen können Viruskapselproteine sein, Adhäsine auf der Spitze von bakteriellen Pili, aber auch lösliche Lektine oder Kohlenhydrat-bindende Domänen von Enzymen oder Toxinen. Die Kohlenhydrat-Bindestellen dieser Proteine sind spezifisch für Glykane auf menschlichem Epithel wie zum Beispiel den Oligosacchariden des ABO- oder des Lewis-Blutgruppensystems. Substanzen, die diese Bindeprozesse modulieren oder blockieren können, sind daher von hohem therapeutischem Interesse bei Infektionskrankheiten. Darüber hinaus können menschliche Lektine, die z. B. bei der Metastasierung von Krebszellen oder bei chronischen Autoimmunerkrankungen Schlüsselrollen einnehmen, in derselben Weise als Zielstrukturen für eine Therapie dienen. Die Forschung und Entwicklung solcher Inhibitoren ist daher von sehr großem Interesse. Dieses Projekt beschäftigt sich mit dem Gebiet der noch in großem Maße unerforschten nicht-Kohlenhydrat Glykomimetika als potentiellen Therapeutika. Solche Glykomimetika haben zahlreiche Vorteile gegenüber bisherigen von Kohlenhydraten abgeleiteten Strukturen z. B. in der Pharmakokinetik und Pharmakodynamik sowie der synthetischen Zugänglichkeit in industriellem Maßstab. Am Beispiel dreier bakterieller Lektine als Zielstrukturen plant das Konsortium aus zwei französischen und zwei deutschen Partnern die Entwicklung eines allgemein anwendbaren Konzepts zur Entwicklung von Lektininhibitoren. In einem Drei-Säulen-Ansatz werden computer-gestützte Verfahren, NMR Fragment-basierte Screeningmethoden und biochemische Verfahren eingesetzt, um insgesamt fünf komplementäre Bibliotheken auf Aktivitäten zu testen. Die resultierenden Hits werden anschließend mittels strukturbasierter Methoden sowie anhand thermodynamischer und kinetischer Parameter charakterisiert und optimiert. Zudem ist eine Präsentation dieser optimierten Leitstrukturen auf multivalenten Trägern, für den Fall nicht hinreichend aktiver monovalenter Leitstrukturen, geplant. Dieses Projekt zielt auf die Entwicklung einer allgemein anwendbaren Methodik im bis dato wissenschaftlich vernachlässigten Bereich der nicht-Kohlenhydrat Lektininhibitoren ab. Nach erfolgreicher Etablierung und Validierung des Prozesses anhand der drei diversen Beispiellektine, soll diese Methodik allgemein anwendbar sein und Verwendung bei anderen mikrobiellen oder humanen Lektinen mit hohem therapeutischem Interesse finden.
Newsroom
Are you interested in a bachelor or master thesis? We are looking forward to your request!