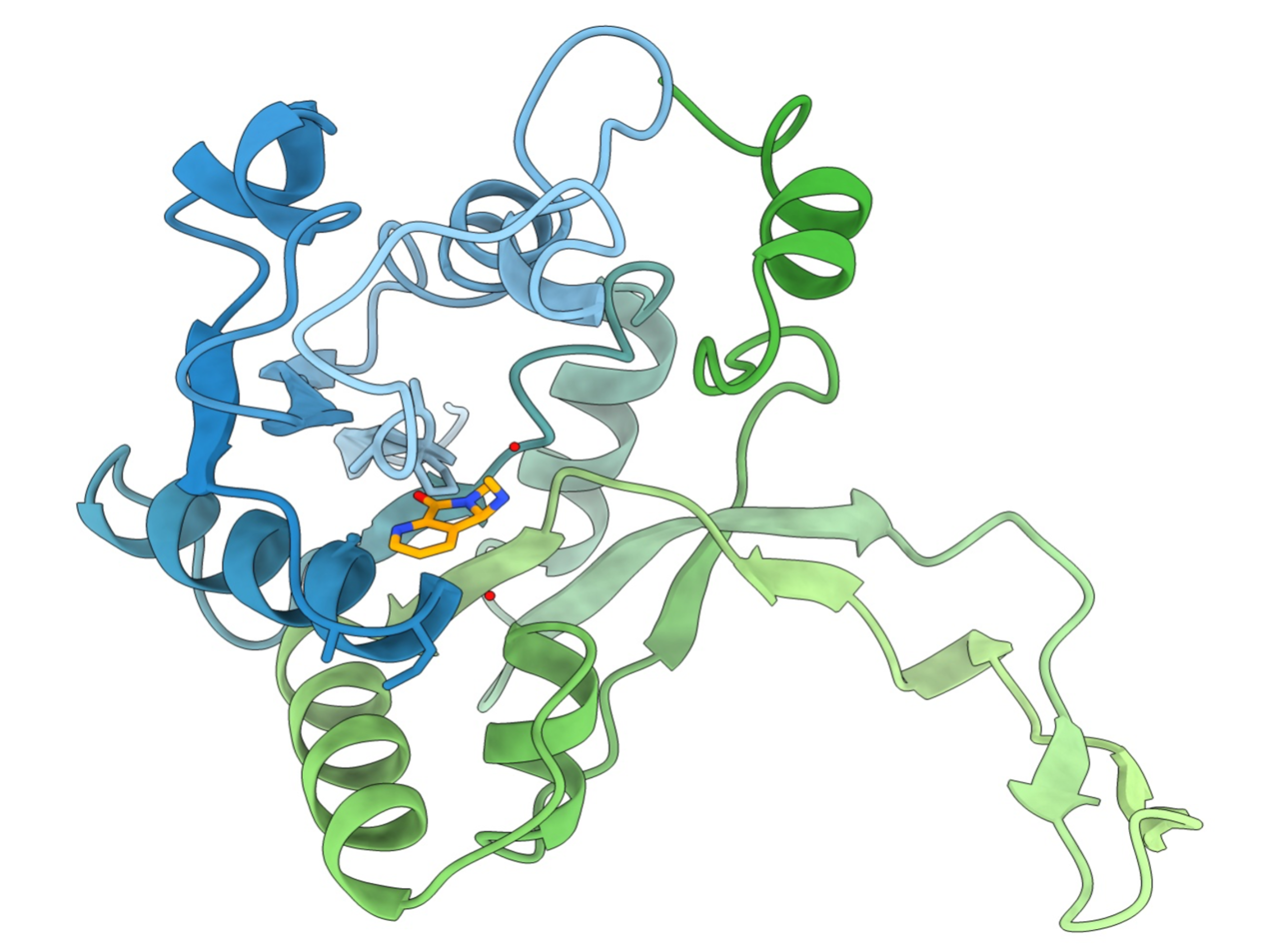
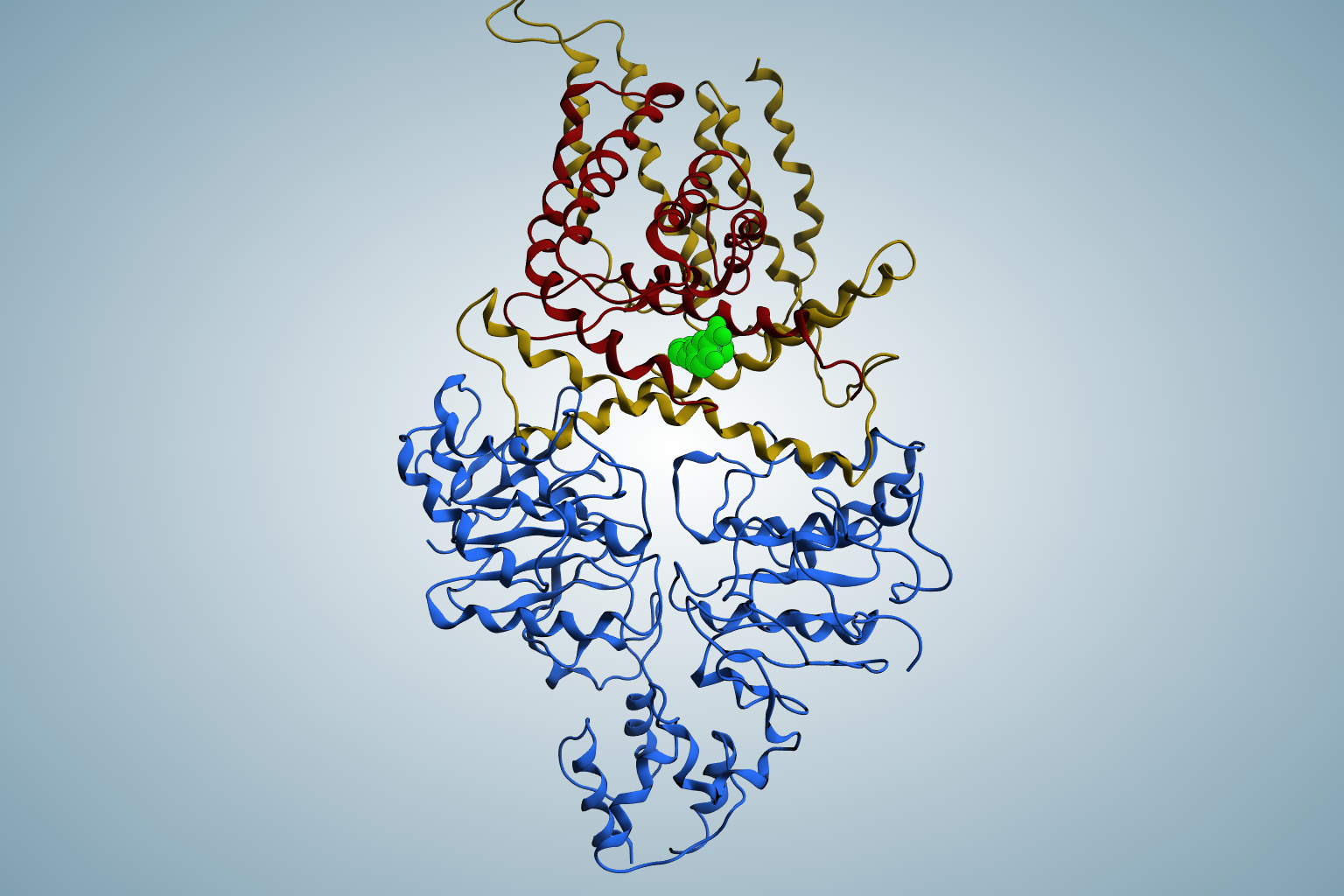
Drug Design and Optimization

Our Research
The first group comprises targets which impair vital mechanisms within the bacteria and effectively kill them. One example is the enzyme DXS which plays a crucial role in the methylerythritol phosphate pathway, which is essential for the biosynthesis of universal isoprenoid precursors in many Gram-negative pathogens, but absent from humans. The second group comprises targets interfering with pathogenicity and virulence without affecting bacterial viability. These pathoblockers are believed to cause a lower rate of resistance development, whilst leaving the commensal microbiota untouched.
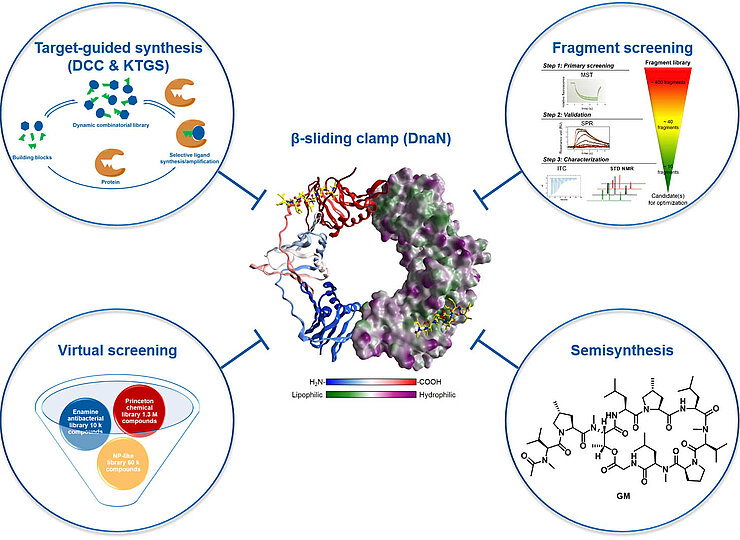
The Hirsch group applies a series of established hit-identification strategies, including structure- and fragment-based drug design, classical medicinal chemistry and virtual screening. In addition, pioneering of innovative protein-templated methods such as dynamic combinatorial chemistry and kinetic target-guided synthesis in terms of the scope of chemical reactions, biological targets and synergistic combinations addresses key bottlenecks. Use of established and innovative techniques to design, synthesize and profile the most promising inhibitors enables efficient subsequent multiparameter optimisation as well as elucidation of the mode of action.
Scientists in the interdisciplinary team have diverse backgrounds such as medicinal chemistry, synthetic organic chemistry, pharmacy, pharmacology, biology or biochemistry, resulting in a diverse skill set.
Our Research
The first group comprises targets which impair vital mechanisms within the bacteria and effectively kill them. One example is the enzyme DXS which plays a crucial role in the methylerythritol phosphate pathway, which is essential for the biosynthesis of universal isoprenoid precursors in many Gram-negative pathogens, but absent from humans. The second group comprises targets interfering with pathogenicity and virulence without affecting bacterial viability. These pathoblockers are believed to cause a lower rate of resistance development, whilst leaving the commensal microbiota untouched.
The Hirsch group applies a series of established hit-identification strategies, including structure- and fragment-based drug design, classical medicinal chemistry and virtual screening. In addition, pioneering of innovative protein-templated methods such as dynamic combinatorial chemistry and kinetic target-guided synthesis in terms of the scope of chemical reactions, biological targets and synergistic combinations addresses key bottlenecks. Use of established and innovative techniques to design, synthesize and profile the most promising inhibitors enables efficient subsequent multiparameter optimisation as well as elucidation of the mode of action.
Scientists in the interdisciplinary team have diverse backgrounds such as medicinal chemistry, synthetic organic chemistry, pharmacy, pharmacology, biology or biochemistry, resulting in a diverse skill set.
Prof Dr Anna K. H. Hirsch

Anna Hirsch read Natural Sciences with a focus on Chemistry at the University of Cambridge and spent her third year at the Massachusetts Institute of Technology, doing a research project with Prof. Timothy Jamison. For her Master’s project, She carried out her Master’s research project in the group of Prof. Steven V. Ley at the University of Cambridge.
She received her Ph.D. from the ETH Zurich in 2008 and worked on the de novo structure-based design and synthesis of inhibitors for an anti-infective target enzyme in the group of Prof. François Diederich. Subsequently, she joined the group of Prof. Jean-Marie Lehn at the Institut de Science et d’Ingénierie Supramoléculaires (ISIS) in Strasbourg as an HFSP postdoctoral fellow, before taking up a position as assistant professor at the Stratingh Institute for Chemistry at the University of Groningen in 2010 where she was promoted to associate professor in 2015.
In 2017, she moved to the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), where she heads the department for drug design and optimization. Her work focuses on anti-infective drug design by adopting rational approaches such as structure- and fragment-based drug design in combination with the target-guided strategies dynamic combinatorial chemistry and kinetic target-guided synthesis.
Anna Hirsch was awarded the Gratama Science Prize in 2014, the SCT-Servier Prize for Medicinal Chemistry in 2015, the Innovation Prize for Medicinal Chemistry of the GdCh/DPhG in 2017, the EFMC Young Medicinal Chemist in Academia Prize (runner-up) in 2019 and in 2024, the RSC-BMCS Capps Green Zomaya Award for Medicinal or Computational Chemistry.
Selected Publications
A. M. Afanasenko, X. Wu, A. de Santi, W. A. M. Elgaher, A. M. Kany, R. Shafiei, M.-S. Schulze, T. F. Schulz, J. Haupenthal, A. K. H. Hirsch*, K. Barta*, Clean Synthetic Strategies to Biologically Active Molecules from Lignin: A Green Path to Drug Discovery. 2024, DOI: 10.1002/anie.202308131
J. Konstantinović, A. M. Kany, A. Alhayek, A. S. Abdelsamie, A. Sikandar, K. Voos, Y. Yao, A. Andreas, R. Shafiei, B. Loretz, E. Schönauer, R. Bals, H. Brandstetter, R. W. Hartmann, C. Ducho, C.-M. Lehr, C. Beisswenger, R. Müller, K. Rox, J. Haupenthal, A. K. H. Hirsch*, Inhibitors of the Elastase LasB for the treatment of Pseudomonas aeruginosa lung infections. 2023, DOI: 10.1021/acscentsci.3c01102
C. Kaya, I. Walter, S. Yahiaoui, A. Sikandar, A. Alhayek, J. Konstantinović, A. M. Kany, J. Haupenthal, J. Köhnke, R. W. Hartmann, A. K. H. Hirsch*, Substrate-Inspired Fragment Merging and Growing Affords Efficacious LasB Inhibitors. 2022, DOI 10.1002/anie.202112295
R. P. Jumde, M. Guardigni, R. M. Gierse, A. Alhayek, Di Zhu, Z. Hamid, S. Johannsen, W. A. M. A. M. Elgaher, P. J. Neusens, C. Nehls, J. Haupenthal, N. Reiling, A. K. H. Hirsch*, Hit-optimization using target-directed dynamic combinatorial chemistry: Development of inhibitors of the anti-infective target 1-deoxy-D-xylulose-5-phosphate synthase. 2021, DOI: 10.1039/D1SC00330E
M. Mondal, N. Radeva, H. Köster, A. Park, C. Potamitis, M. Zervou, G. Klebe*, A. K. H. Hirsch*, Structure-based design of inhibitors of the aspartic protease endothiapepsin by exploiting dynamic combinatorial chemistry. 2014, DOI: 10.1002/anie.201309682
A complete list of publications can be found on the HIPS website
Technology Offers
The following technologies have been developed and patented by the department Drug Design and Optimization:
Biodynamers enhance drug delivery
Innovative Inhibitors of Pseudomonas Pathogenicity
Cystobactamides – novel antibacterials against gram-negative pathogens
Overcoming resistance - Novel inhibitors of virulence factor LasB of Pseudomonas aeruginosa
Overcoming resistance - Innovative broad-spectrum inhibitors of Metallo- β-lactamases
Research Projects
Development of molecules that interfere with the PQS quorum sensing communication system in Pseudomonas aeruginosa
The department is working on the development of compounds interfering with the PQS quorum sensing cell-to-cell-communication system in Pseudomonas aeruginosa. Lung infections resulting from this opportunistic ESKAPE pathogen are regarded as one of the major causes of morbidity and mortality in patients with cystic fibrosis. Resistance to generally applied antibiotics is often associated with biofilm formation.
Both the formation of biofilms as well as the production of virulence factors is regulated by a complex quorum sensing communication network. Within this network, bacteria coordinate population-wide behaviours in a cell-density-dependent manner. In addition to the quorum sensing networks LasR and RhlR, which are present in numerous bacteria, P. aeruginosa uses a third communication system. This system is regulated by PQS (Pseudomonas Quinoline Signal, 2-heptyl-3-hydroxy-4-quinolone) as an intercellular signalling molecule.
The overall aim of the project is to discover and advance compounds for the treatment of P. aeruginosa infections. These anti-infective agents interfere with PQS quorum sensing communication in order to prevent biofilm formation and to reduce the production of multiple quorum sensing-regulated virulence factors without affecting bacterial viability. This novel anti-infective strategy circumvents usual resistance mechanisms and could lead to low rates of resistance development, while the pathogen-specific mode-of-action preserves the commensal (beneficial) microbiota.
Newsroom
Are you interested in a bachelor or master thesis? We are looking forward to your request!

![[Translate to English:] 3D-kontrastierte Oberflächenstruktur von LasB (beige) mit einem Inhibitor der 3. Generation (dunkelblau), der Zink (grau) bindet.](/fileadmin/_processed_/8/b/csm_LasB_Foto_b7734ae7a4.png)