Experimental Virology

Our Research
Overview of our key projects:
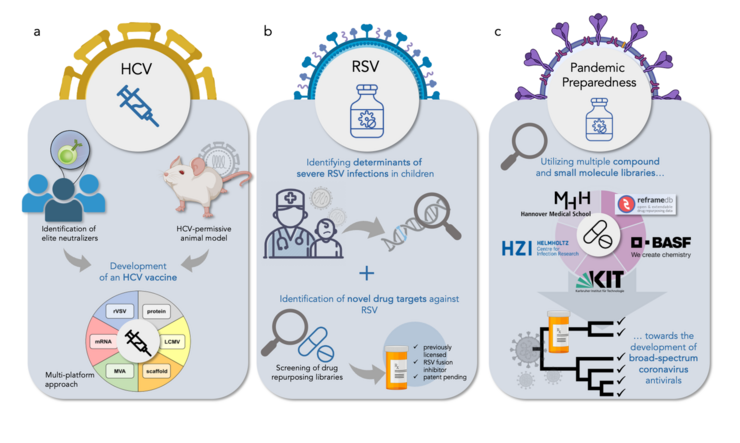
- a. Identification of elite neutralizers in HCV infections and establishment of an HCV-permissive animal model to aid in the development of an HCV vaccine. The HCV vaccine is being developed using a multi-platform strategy.
- b. Identifying modifiers of RSV disease severity in infants without pre-diseases to aid us in the identification of genetic variants and/or quantitative biomarkers that will predict the risk for severe RSV disease. Moreover, we utilize drug repurposing libraries to identify novel drug targets against RSV. As an example, we have successfully identified Lonafarnib as a RSV fusion inhibitor using this method.
- c. Screening of multiple libraries composed of licensed drugs for the discovery of host-targeting antivirals. Ultimately, we aim to identify broad-spectrum coronavirus antivirals in light of pandemic preparedness.
Hepatitis C Virus (HCV) Research
According to estimates by the World Health Organization (WHO), around 58 million people are chronically infected with the hepatitis C virus (HCV). As a result of the chronic infection, many patients develop inflammation of the liver (hepatitis), which can damage the function of the organ and lead to fibrosis, cirrhosis, and liver cancer. Fortunately, chronic hepatitis C is now very treatable with combination therapy. However, major challenges remain: The vast majority of HCV-infected individuals are undiagnosed and remain undetected due to the usually slow progression of the disease. In addition, a drug cure does not protect against re-infection with HCV. Up to 1.5 million people become newly infected with the virus every year. Therefore, developing a prophylactic vaccine to limit viral transmission and ensure treatment success remains crucial.
We investigate the principles responsible for immune protection against HCV, concentrating primarily on antibody-mediated mechanisms. We examine the strategies used by viruses to evade the immune response and develop cell culture and animal models to test the efficacy of vaccine candidates. These systems are used to source proprietary vaccine candidates for clinical development.
Respiratory Syncytial Virus (RSV) research
Respiratory syncytial virus (RSV), like HCV, has a worldwide distribution. In young healthy adults, an RSV infection usually presents as a mild cold. However, RSV can also cause severe lower respiratory tract infections. Young children, immunocompromised patients such as transplant recipients, and adults >60 years of age are particularly at risk. Worldwide, RSV causes alone 33.4 million cases of acute lower respiratory tract infections in young children and between 53,000 and 199,000 associated deaths per year. Why some children develop particularly severe courses of disease is not well understood. Currently, only a few treatment options are available. Recently two vaccines were approved for individuals aged 60 and older for the prevention of lower respiratory tract disease caused by RSV and one of them is additionally lisenced for vaccination of pregnant women.
Our research focuses on the principles responsible for severe RSV infections in young children in order to develop diagnostic methods to better protect these particularly susceptible children. In addition, we investigate direct acting antiviral inhibitors against RSV.
Pandemic Preparedness
Up until mid-2023, the pandemic COVID-19 agent, SARS-CoV-2, had killed almost 7 million people worldwide. Though several vaccines are available, only a few treatment options for treating SARS-CoV-2 infections are available. In light of pandemic preparedness, we anticipate other coronaviruses will transmit to humans in the future. Therefore, we are developing antiviral molecules that are effective against a plethora of various coronaviruses.
For information on current projects of the research group Experimental Virology please visit the TWINCORE website.
Our Research
Overview of our key projects:
- a. Identification of elite neutralizers in HCV infections and establishment of an HCV-permissive animal model to aid in the development of an HCV vaccine. The HCV vaccine is being developed using a multi-platform strategy.
- b. Identifying modifiers of RSV disease severity in infants without pre-diseases to aid us in the identification of genetic variants and/or quantitative biomarkers that will predict the risk for severe RSV disease. Moreover, we utilize drug repurposing libraries to identify novel drug targets against RSV. As an example, we have successfully identified Lonafarnib as a RSV fusion inhibitor using this method.
- c. Screening of multiple libraries composed of licensed drugs for the discovery of host-targeting antivirals. Ultimately, we aim to identify broad-spectrum coronavirus antivirals in light of pandemic preparedness.

Hepatitis C Virus (HCV) Research
According to estimates by the World Health Organization (WHO), around 58 million people are chronically infected with the hepatitis C virus (HCV). As a result of the chronic infection, many patients develop inflammation of the liver (hepatitis), which can damage the function of the organ and lead to fibrosis, cirrhosis, and liver cancer. Fortunately, chronic hepatitis C is now very treatable with combination therapy. However, major challenges remain: The vast majority of HCV-infected individuals are undiagnosed and remain undetected due to the usually slow progression of the disease. In addition, a drug cure does not protect against re-infection with HCV. Up to 1.5 million people become newly infected with the virus every year. Therefore, developing a prophylactic vaccine to limit viral transmission and ensure treatment success remains crucial.
We investigate the principles responsible for immune protection against HCV, concentrating primarily on antibody-mediated mechanisms. We examine the strategies used by viruses to evade the immune response and develop cell culture and animal models to test the efficacy of vaccine candidates. These systems are used to source proprietary vaccine candidates for clinical development.
Respiratory Syncytial Virus (RSV) research
Respiratory syncytial virus (RSV), like HCV, has a worldwide distribution. In young healthy adults, an RSV infection usually presents as a mild cold. However, RSV can also cause severe lower respiratory tract infections. Young children, immunocompromised patients such as transplant recipients, and adults >60 years of age are particularly at risk. Worldwide, RSV causes alone 33.4 million cases of acute lower respiratory tract infections in young children and between 53,000 and 199,000 associated deaths per year. Why some children develop particularly severe courses of disease is not well understood. Currently, only a few treatment options are available. Recently two vaccines were approved for individuals aged 60 and older for the prevention of lower respiratory tract disease caused by RSV and one of them is additionally lisenced for vaccination of pregnant women.
Our research focuses on the principles responsible for severe RSV infections in young children in order to develop diagnostic methods to better protect these particularly susceptible children. In addition, we investigate direct acting antiviral inhibitors against RSV.
Pandemic Preparedness
Up until mid-2023, the pandemic COVID-19 agent, SARS-CoV-2, had killed almost 7 million people worldwide. Though several vaccines are available, only a few treatment options for treating SARS-CoV-2 infections are available. In light of pandemic preparedness, we anticipate other coronaviruses will transmit to humans in the future. Therefore, we are developing antiviral molecules that are effective against a plethora of various coronaviruses.
For information on current projects of the research group Experimental Virology please visit the TWINCORE website.
Prof Dr Thomas Pietschmann
At EVIR, we focus on bridging the gap between fundamental research and clinical practice. We study the molecular mechanisms of viral diseases, such as RSV and HCV, always keeping clinical questions in mind, ensuring a seamless approach from bench to bedside and back again.

Thomas Pietschmann, born in Würzburg, studied biology at the Justus Maximilian University of Würzburg and Duke University, Durham USA. In 2000, he received his doctorate at the Institute of Virology at the University of Würzburg on mechanisms of virus morphogenesis in retroviruses and joined Professor Ralf Bartenschlager's group at the Institute of Virology in Mainz as a postdoc. In 2002 he moved to the Department of Molecular Virology at Heidelberg University together with Bartenschlager. In 2006 Thomas Pietschmann founded an Emmy Noether junior research group that focused on the morphogenesis and entry mechanism of the hepatitis C virus. In spring 2007 he and his working group were appointed to TWINCORE. Since 2012 he is head of the Department of Experimental Virology at TWINCORE and has been spokesman for the Helmholtz program “Infection Research” since 2021. In 2023 he joined the HZI management team as scientific co-director and was elected to the executive board of the German Center for Infection Research (DZIF).
Selected Publications
Sake SM, Zhang X, Rajak MK, Urbanek-Quaing M, Carpentier A, Gunesch AP, Grethe C, Matthaei A, Rückert J, Galloux M, Larcher T, Le Goffic R, Hontonnou F, Chatterjee AK, Johnson K, Morwood K, Rox K, Elgaher WAM, Huang J, Wetzke M, Hansen G, Fischer N, Eléouët JF, Rameix-Welti MA, Hirsch AKH, Herold E, Empting M, Lauber C, Schulz TF, Krey T, Haid S, Pietschmann T. Drug repurposing screen identifies lonafarnib as respiratory syncytial virus fusion protein inhibitor. Nat Commun. 2024 Feb 8;15(1):1173. doi: 10.1038/s41467-024-45241-y. PMID: 38332002; PMCID: PMC10853176.
Weber T, Potthoff J, Bizu S, Labuhn M, Dold L, Schoofs T, Horning M, Ercanoglu MS, Kreer C, Gieselmann L, Vanshylla K, Langhans B, Janicki H, Ströh LJ, Knops E, Nierhoff D, Spengler U, Kaiser R, Bjorkman PJ, Krey T, Bankwitz D, Pfeifer N, Pietschmann T, Flyak AI, Klein F. Analysis of antibodies from HCV elite neutralizers identifies genetic determinants of broad neutralization. Immunity. 2022 Feb 8;55(2):341-354.e7. doi: 10.1016/j.immuni.2021.12.003. Epub 2022 Jan 5.
Risso-Ballester J, Galloux M, Cao J, Le Goffic R, Hontonnou F, Jobart-Malfait A, Desquesnes A, Sake SM, Haid S, Du M, Zhang X, Zhang H, Wang Z, Rincheval V, Zhang Y, Pietschmann T, Eléouët JF, Rameix-Welti MA, Altmeyer R. A condensate-hardening drug blocks RSV replication in vivo. Nature. 2021 Jul;595(7868):596-599. doi: 10.1038/s41586-021-03703-z. Epub 2021 Jul 7. PMID: 34234347.
Bankwitz D, Bahai A, Labuhn M, Doepke M, Ginkel C, Khera T, Todt D, Ströh LJ, Dold L, Klein F, Klawonn F, Krey T, Behrendt P, Cornberg M, McHardy AC, Pietschmann T. Hepatitis C reference viruses highlight potent antibody responses and diverse viral functional interactions with neutralising antibodies. Gut. 2021 Sep;70(9):1734-1745. doi: 10.1136/gutjnl-2020-321190. Epub 2020 Dec 15.
Bankwitz D, Doepke M, Hueging K, Weller R, Bruening J, Behrendt P, Lee JY, Vondran FWR, Manns MP, Bartenschlager R, Pietschmann T. Maturation of secreted HCV particles by incorporation of secreted ApoE protects from antibodies by enhancing infectivity. J Hepatol. 2017 Sep;67(3):480-489. doi: 10.1016/j.jhep.2017.04.010. Epub 2017 Apr 22.
Find more publications here.