
Our Research
The research led by Lina Herhaus in the research group “Immune Signaling” (IMSI) focuses on understanding how the immune system detects and responds to threats such as infections and resulting inflammation. By exploring the molecular mechanisms that regulate immune signaling pathways, the group aims to uncover how cells maintain protein homeostasis and safeguard against pathogenic challenges. This work has far-reaching implications for public health, as it addresses fundamental questions about how the body combats infections and avoids immune dysfunction.
At the heart of their research is the investigation of post-translational modifications and how these molecular "switches" influence immune signaling and cellular quality control. One of the group’s key aims is to understand how immune cells recognize and degrade misfolded or damaged proteins to ensure cellular health—a process critical for fighting infections and preventing diseases. The group also studies how pathogens exploit or evade these processes to establish infections and how host cells counteract these strategies.
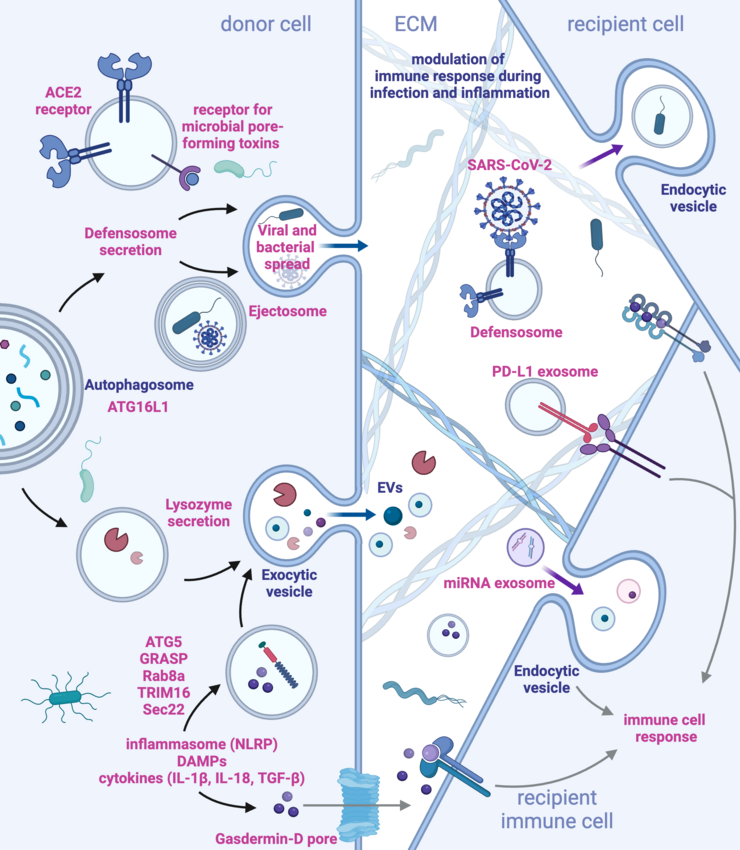
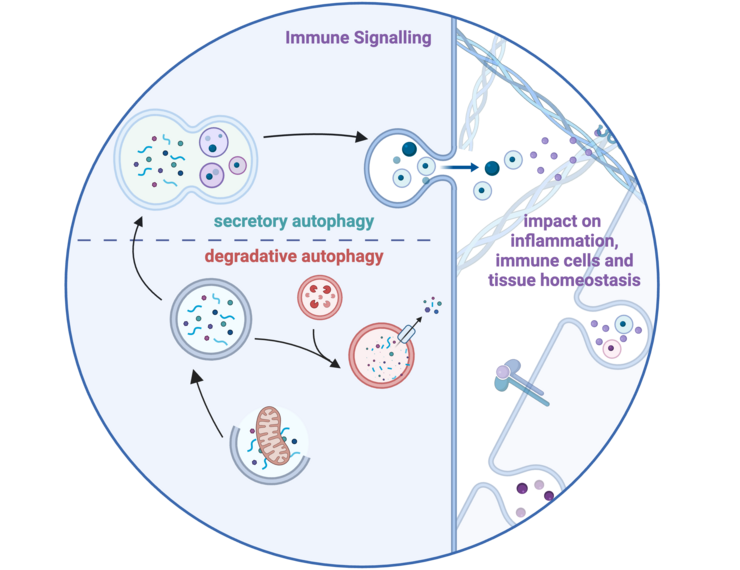
A major focus is the role of autophagy and vesicle trafficking in immune regulation. The team is particularly interested in how these pathways contribute to immune cell activation and pathogen clearance. Using cutting-edge technologies, including proteomics, imaging, and functional genomics, the group investigates the interplay between immune signaling and cellular metabolism. Their research seeks to identify key molecular players, such as the protein IRGQ, which could act as regulators of immune quality control and potential therapeutic targets.
The Immune Signaling Group’s work is highly relevant to society, as it informs the development of new treatments for infectious diseases and inflammatory disorders. By uncovering the mechanisms that underpin immune responses, their research has the potential to drive innovative therapies that harness the immune system for combating diseases. This knowledge could lead to the development of precision medicine approaches, benefiting patients with conditions ranging from chronic infections to tumor growth. In this way, the group contributes to advancing both basic scientific understanding and translational medical research for the betterment of public health.
Our Research
The research led by Lina Herhaus in the research group “Immune Signaling” (IMSI) focuses on understanding how the immune system detects and responds to threats such as infections and resulting inflammation. By exploring the molecular mechanisms that regulate immune signaling pathways, the group aims to uncover how cells maintain protein homeostasis and safeguard against pathogenic challenges. This work has far-reaching implications for public health, as it addresses fundamental questions about how the body combats infections and avoids immune dysfunction.
At the heart of their research is the investigation of post-translational modifications and how these molecular "switches" influence immune signaling and cellular quality control. One of the group’s key aims is to understand how immune cells recognize and degrade misfolded or damaged proteins to ensure cellular health—a process critical for fighting infections and preventing diseases. The group also studies how pathogens exploit or evade these processes to establish infections and how host cells counteract these strategies.
A major focus is the role of autophagy and vesicle trafficking in immune regulation. The team is particularly interested in how these pathways contribute to immune cell activation and pathogen clearance. Using cutting-edge technologies, including proteomics, imaging, and functional genomics, the group investigates the interplay between immune signaling and cellular metabolism. Their research seeks to identify key molecular players, such as the protein IRGQ, which could act as regulators of immune quality control and potential therapeutic targets.
The Immune Signaling Group’s work is highly relevant to society, as it informs the development of new treatments for infectious diseases and inflammatory disorders. By uncovering the mechanisms that underpin immune responses, their research has the potential to drive innovative therapies that harness the immune system for combating diseases. This knowledge could lead to the development of precision medicine approaches, benefiting patients with conditions ranging from chronic infections to tumor growth. In this way, the group contributes to advancing both basic scientific understanding and translational medical research for the betterment of public health.
Dr Lina Herhaus
Understanding cellular communication is key to fighting infections. Our research uncovers molecular insights to transform health and disease.

Lina Herhaus is an accomplished scientist specializing in biochemistry, cell signaling, and immune research. Since October 2024, she has lead the "Immune Signaling" group at the Helmholtz Centre for Infection Research(HZI), funded by the MICROSTAR Program, and has served as a W1 Professor at Hannover Medical School (MHH). Previously, she led the Immune Signaling Group at Goethe University Frankfurt (2020 - 2024), advancing the understanding of immune pathways and molecular mechanisms.
Lina’s research career reflects her passion for exploring complex cellular processes. From 2019 to 2020, she worked as a staff scientist at the Frankfurt Cancer Institute, specializing in cellular and biochemical analysis. Before that, she received an EMBO Long-Term Fellowship and performed her Postdoctoral work in Prof. Ivan Dikic’s lab at the Institute for Biochemistry II, Frankfurt (2015 - 2019), where she deepened her expertise in protein signaling and autophagy.
Lina earned her PhD in Biochemistry and Cell Signaling in 2014 at the MRC in Dundee under Dr. Gopal Sapkota, exploring intricate molecular pathways. She holds a double degree in Molecular Biology and Applied Biology (first-class honors, 2010) from Hochschule Bonn-Rhein-Sieg, Germany, and the University of Dundee. Her early career included a research stay at ECOSUR, Mexico (2006–2007).
Her contributions to science have been widely recognized. In 2024, she received the Signal Transduction Society (STS) Science Award and joined the AIM (Autophagy, Inflammation & Metabolism) Council of International Rising Stars. Earlier, she won the Tim Hunt Prize for Cell Biology (2013) for her innovative advances in basic research.
Team








Publications
Selected Publications
- Herhaus, L., Gestal-Mato, U., Eapen, V. V., Macinkovic, I., Bailey, H. J., Prieto Garcia, C., Misra, M., Jacomin, A. C., Ammanath, A. V., Covarrubias-Pinto, A., Michaelis, J., Bagaric, I., Zöllner, J., Bhaskara, R.M., Bündgen, G., Husnjak, K., Vollrath, J., Gikandi, A., Ribicic, S., Langer, J., Bopp, T., Weigert, A., Mancias, J., Harper, J.W., and Dikic, I.(2024). IRGQ-mediated autophagy in MHC-I quality control promotes tumor immune evasion. Cell. https://doi.org/10.1016/J.CELL.2024.09.048.
- Weigert, A., and Herhaus, L. (2023). Immune modulation through secretory autophagy. J Cell Biochem. 10.1002/JCB.30427. https://onlinelibrary.wiley.com/doi/full/10.1002/jcb.30427
- Gestal-Mato, U., and Herhaus, L. (2023). Autophagy-dependent regulation of MHC-I molecule presentation. J Cell Biochem. 10.1002/JCB.30416. https://onlinelibrary.wiley.com/doi/10.1002/jcb.30416
- Herhaus, L. (2021). TBK1 (TANK-binding kinase 1)-mediated regulation of autophagy in health and disease. Matrix Biol 100–101, 84–98. 10.1016/j.matbio.2021.01.004. https://www.sciencedirect.com/science/article/pii/S0945053X21000056
- Herhaus, L., Bhaskara, R.M., (…), Dikic, I. (2020). TBK1-mediated phosphorylation of LC3C and GABARAP-L2 controls autophagosome shedding by ATG4 protease. EMBO Rep 21, e48317. 10.15252/embr.201948317. https://www.embopress.org/doi/full/10.15252/embr.201948317
Projects
Autophagy mechanisms in intercellular sensing of tissue homeostasis by the immune system
Disturbance of tissue homeostasis triggers immune responses to restore balance. Non-canonical autophagy, crucial for cellular homeostasis, and its sensing by the immune system remain poorly understood. This project investigates how autophagy impacts immune responses, focusing on MHC-I-like molecule regulation and secretory autophagy's role in extracellular vesicle (EV) secretion. Using genetic screens, high-parameter imaging, automated microscopy, and Omics approaches in murine and human models, we aim to uncover how autophagy-driven processes influence immune-dependent tissue homeostasis during normal and disturbed conditions.
Funding: DFG via SFB1177
Boehringer Ingelheim Exploration Grant project
Our new Boehringer Ingelheim Exploration Grant project explores how membrane contact sites (MCS) shape cellular defense mechanisms during bacterial infection. MCS act as key communication hubs between organelles, yet their contribution to infection biology remains largely unknown. Emerging evidence indicates that intracellular pathogens such as Salmonella exploit these structures to manipulate host cell processes and secure their survival. Using a high-content, microscopy-based siRNA screen targeting human trafficking proteins, we aim to identify the host factors that facilitate Salmonella’s intracellular niche formation and nutrient acquisition. Validations using CRISPR gene editing and functional assays will define the molecular mechanisms underlying these interactions. This exploratory study has the potential to reveal novel host-pathogen interfaces and therapeutic targets.