Innovative Organoid Research

Our Research
Throughout evolution, all organisms, from viruses and single-celled microorganisms to humans, have developed mechanisms at various levels to protect themselves from internal and external disruptions. These resilience mechanisms enable organisms to successfully respond to changes, inhabit their environments, and explore new ones. The genetic conflict between pathogens and their hosts significantly drives these evolutionary changes. A key role in this pathogen-host conflict is played by so-called effector proteins, which influence the functions of host cells to favour disease progression and evade immune responses.
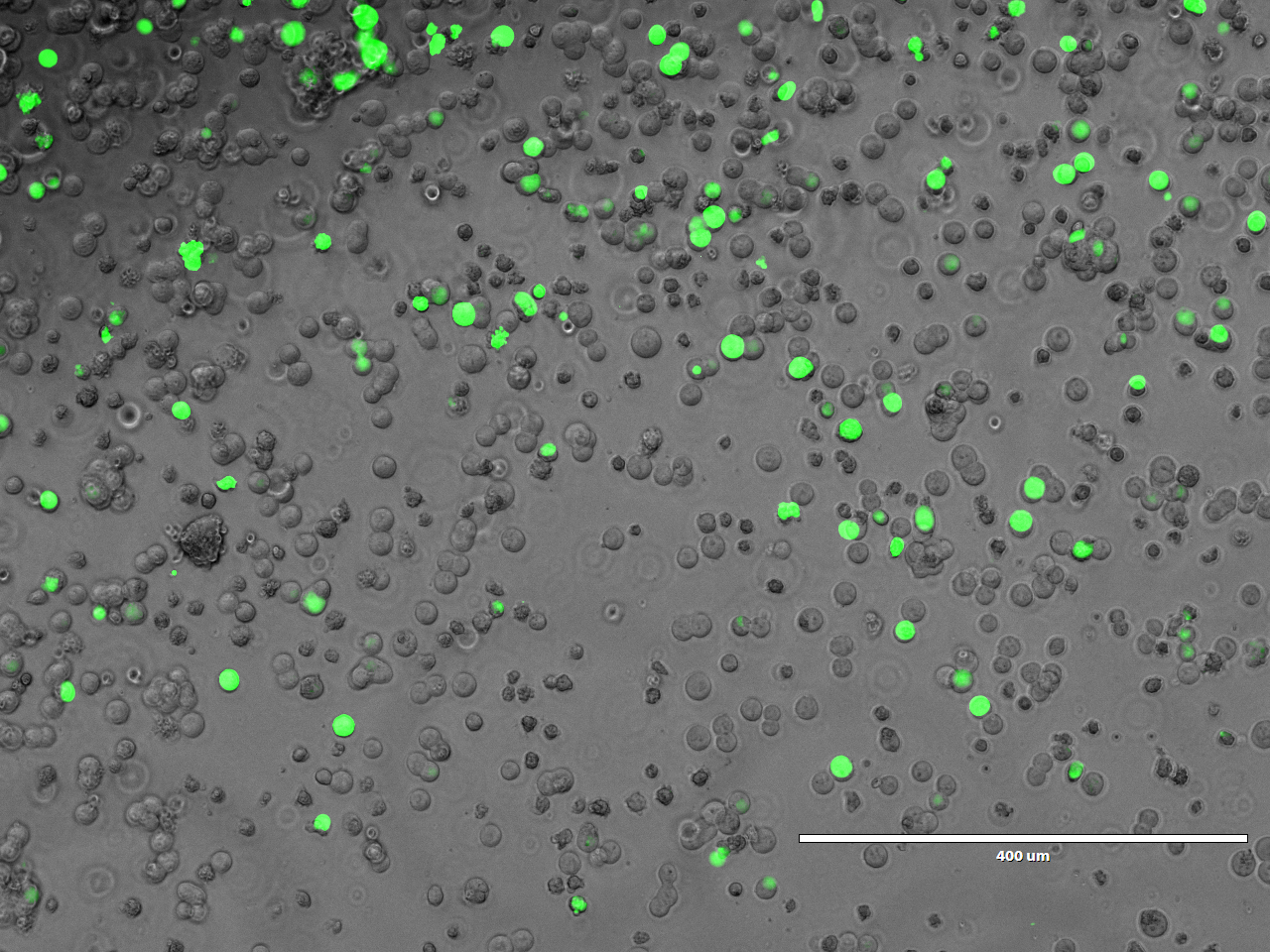
Our aim is to gain insights into host-pathogen interactions and thereby close existing knowledge gaps by creating a unique collection of open reading frames (ORFs) of pathogenic viruses, bacteria, and parasites. This collection allows for comprehensive screening of effector proteins in various human and non-human cell lines using different reporter assays. This facilitates the systematic identification of effector functions. Additionally, our work in this field enables the preventive characterization of effectors from disease-causing pathogens with pandemic potential, such as coronaviruses and other respiratory viruses.
To combat infectious diseases, lectins represent a promising tool as they can bind to the surface of microorganisms such as viruses, bacteria, and fungi, thereby influencing their attachment to host cells. Lectins are proteins that specifically bind to carbohydrates and are found in a variety of organisms, including humans. By inhibiting the binding of pathogens to host cells, lectins potentially prevent or at least mitigate infection. Currently, we are working on producing a comprehensive library of human and rodent-derived lectins, which will enable a wide range of in vitro and in vivo screening applications.
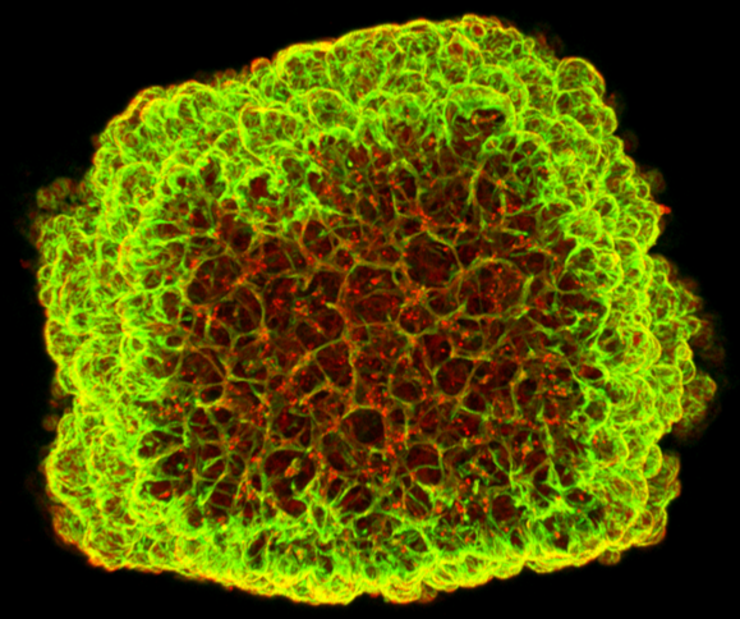
Our organoid platform serves as a basis for exploring the potential of lectins and studying effector proteins. Moreover, it facilitates research on infections and the evaluation of vaccines and innovative therapies. In this regard, we additionally employ CRISPR technology to genetically modify organoids, thereby enabling the study of unique and clinically relevant biological processes. By producing disease-specific organoids from patient samples, we promote the understanding of diseases and the development of treatment methods. Our research is supported by state-of-the-art robotic systems for automating cell culture processes, allowing for optimized and reproducible production of organoids in high throughput.
By integrating innovative technologies and creating a globally unique collection of effector proteins and lectins, we aim to provide new impulses for the exploration of interactions between hosts and pathogens.
Our Research
Throughout evolution, all organisms, from viruses and single-celled microorganisms to humans, have developed mechanisms at various levels to protect themselves from internal and external disruptions. These resilience mechanisms enable organisms to successfully respond to changes, inhabit their environments, and explore new ones. The genetic conflict between pathogens and their hosts significantly drives these evolutionary changes. A key role in this pathogen-host conflict is played by so-called effector proteins, which influence the functions of host cells to favour disease progression and evade immune responses.
Our aim is to gain insights into host-pathogen interactions and thereby close existing knowledge gaps by creating a unique collection of open reading frames (ORFs) of pathogenic viruses, bacteria, and parasites. This collection allows for comprehensive screening of effector proteins in various human and non-human cell lines using different reporter assays. This facilitates the systematic identification of effector functions. Additionally, our work in this field enables the preventive characterization of effectors from disease-causing pathogens with pandemic potential, such as coronaviruses and other respiratory viruses.
To combat infectious diseases, lectins represent a promising tool as they can bind to the surface of microorganisms such as viruses, bacteria, and fungi, thereby influencing their attachment to host cells. Lectins are proteins that specifically bind to carbohydrates and are found in a variety of organisms, including humans. By inhibiting the binding of pathogens to host cells, lectins potentially prevent or at least mitigate infection. Currently, we are working on producing a comprehensive library of human and rodent-derived lectins, which will enable a wide range of in vitro and in vivo screening applications.
Our organoid platform serves as a basis for exploring the potential of lectins and studying effector proteins. Moreover, it facilitates research on infections and the evaluation of vaccines and innovative therapies. In this regard, we additionally employ CRISPR technology to genetically modify organoids, thereby enabling the study of unique and clinically relevant biological processes. By producing disease-specific organoids from patient samples, we promote the understanding of diseases and the development of treatment methods. Our research is supported by state-of-the-art robotic systems for automating cell culture processes, allowing for optimized and reproducible production of organoids in high throughput.
By integrating innovative technologies and creating a globally unique collection of effector proteins and lectins, we aim to provide new impulses for the exploration of interactions between hosts and pathogens.
Prof. Josef Penninger
There are still many unexpected cellular targets of microbial pathogens and their molecular mechanisms to discover.

Josef Penninger was born on 5 September 1964 in Gurten, Oberösterreich. After attending medical school in Innsbruck, he started his scientific career at the University of Toronto, Canada. In 2002, he was the founding director of the Institute for Molecular Biotechnology IMBA – a research institute of the Austrian Academy of Sciences in Vienna. In 2018, he returned to Canada as a Canada 150 Chair and took over the Life Sciences Institute at the University of British Columbia in Vancouver. Starting from 1 July 2023, he has been appointed as Scientific Director of the HZI in Braunschweig. In 2024, he completed his PhD in Medical Science at Tokyo Medical and Dental University in Japan.
Since 1995 Josef Penninger has been receiving multiple competitive research grants in the EU and North America, among those a C150 Canada Research Chair in Functional Genetics, an Innovator Award from the US, an EU Excellence Grant, as well as an ERC Advanced Grant.
Josef Penninger has earned a long list of awards and honorary awards. Among those are the Ernst-Jung-Price for Medicine, the Descartes-Price (highest European award) and the Wittgenstein-Price, also referred to as the “Austrian Nobel Price”. In 2015 he placed 11th in the ranking of the most influential thought leaders in German-speaking countries. Additionally Penninger has been given an Honorary Professor title of the Chinese University of Qingdao and is a recipient of the Austrian Cross of Merit. He is regularly distinguished for his publications and citation rates, e.g. as Highly-cited Researcher by Clarivate, Top-Cited Scholar by Scilit and most-cited developmental biologist in the German-speaking countries, and he was among the Top 10 of the most cited scientists worldwide twice.
Team
















Selected Publications
Frenz-Wiessner S, Fairley SD, Buser M, Goek I, Salewskij K, Jonsson G, Illig D, Zu Putlitz B, Petersheim D, Li Y, Chen PH, Kalauz M, Conca R, Sterr M, Geuder J, Mizoguchi Y, Megens RTA, Linder MI, Kotlarz D, Rudelius M, Penninger JM, Marr C, Klein C. Generation of complex bone marrow organoids from human induced pluripotent stem cells. Nat Methods. 2024 Feb 19. doi: 10.1038/s41592-024-02172-2. Epub ahead of print. PMID: 38374263.
Quintard C, Tubbs E, Jonsson G, Jiao J, Wang J, Werschler N, Laporte C, Pitaval A, Bah TS, Pomeranz G, Bissardon C, Kaal J, Leopoldi A, Long DA, Blandin P, Achard JL, Battail C, Hagelkruys A, Navarro F, Fouillet Y, Penninger JM, Gidrol X. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat Commun. 2024 Feb 16;15(1):1452. doi: 10.1038/s41467-024-45710-4. PMID: 38365780; PMCID: PMC10873332.
Batlle D, Monteil V, Garreta E, Hassler L, Wysocki J, Chandar V, Schwartz RE, Mirazimi A, Montserrat N, Bader M, Penninger JM. Evidence in favor of the essentiality of human cell membrane-bound ACE2 and against soluble ACE2 for SARS-CoV-2 infectivity. Cell. 2022 May 26;185(11):1837-1839. doi: 10.1016/j.cell.2022.05.004. PMID: 35623327; PMCID: PMC9134488.
Zoufaly A, Poglitsch M, Aberle JH, Hoepler W, Seitz T, Traugott M, Grieb A, Pawelka E, Laferl H, Wenisch C, Neuhold S, Haider D, Stiasny K, Bergthaler A, Puchhammer-Stoeckl E, Mirazimi A, Montserrat N, Zhang H, Slutsky AS, Penninger JM. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020 Nov;8(11):1154-1158. doi: 10.1016/S2213-2600(20)30418-5. Epub 2020 Sep 24. Erratum in: Lancet Respir Med. 2020 Nov;8(11):e78. PMID: 33131609; PMCID: PMC7515587.
Chabloz A, Schaefer JV, Kozieradzki I, Cronin SJF, Strebinger D, Macaluso F, Wald J, Rabbitts TH, Plückthun A, Marlovits TC, Penninger JM. Salmonella-based platform for efficient delivery of functional binding proteins to the cytosol. Commun Biol. 2020 Jul 3;3(1):342. doi: 10.1038/s42003-020-1072-4. PMID: 32620833; PMCID: PMC7335062.