Molecular Cell Biology

Our Research
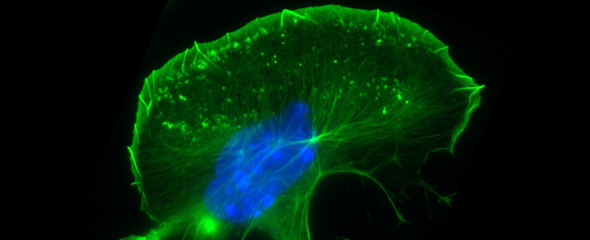
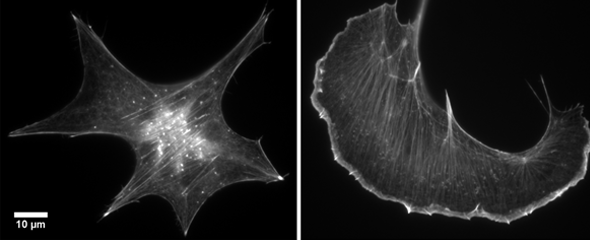
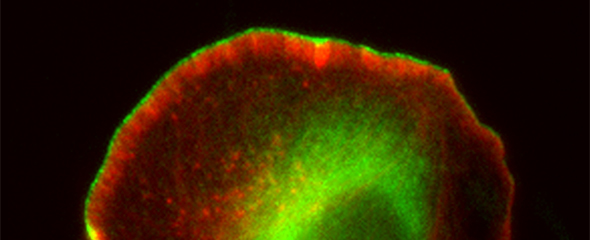
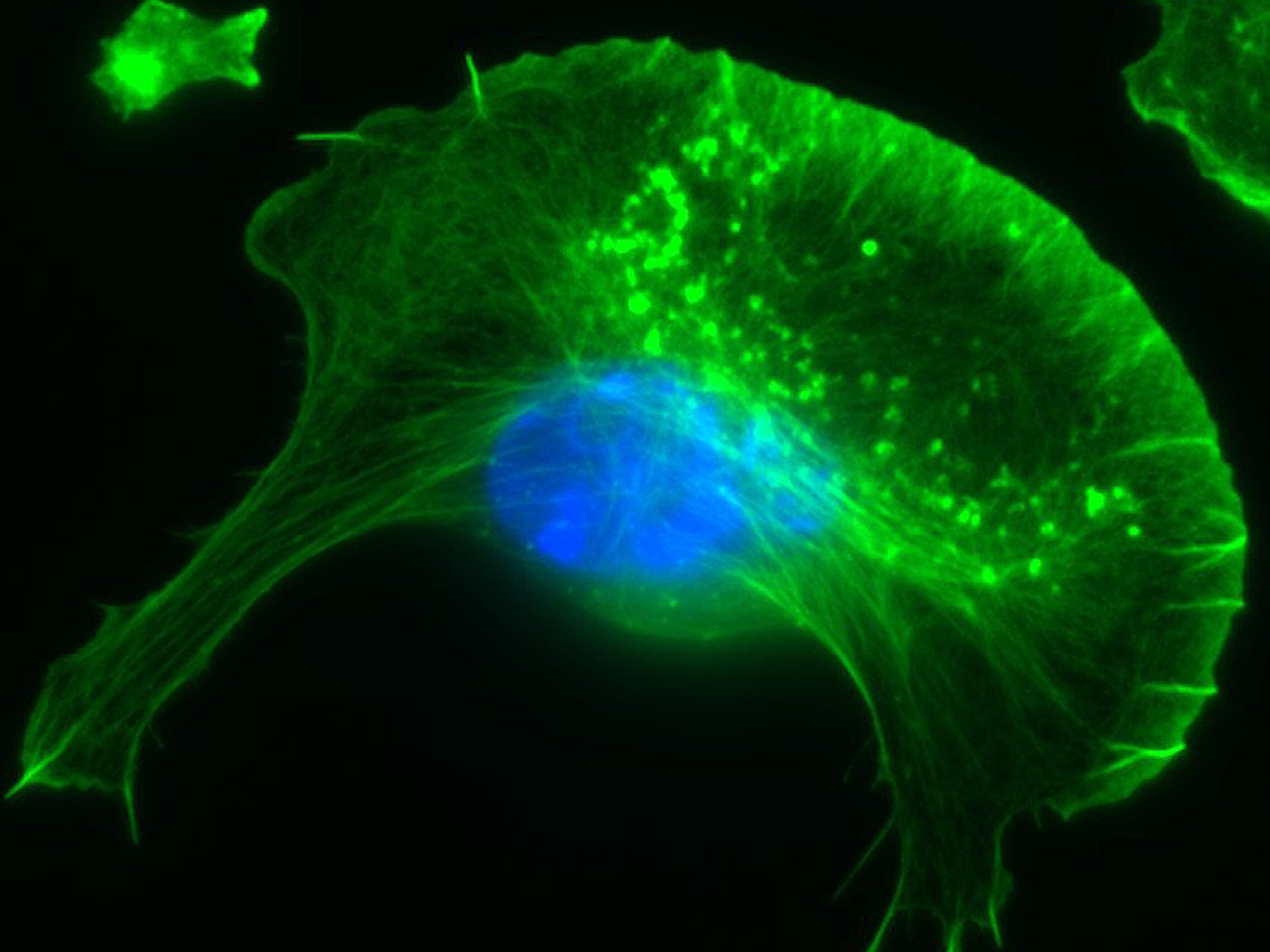
Our group is mainly interested in understanding the molecular mechanisms that drive the reorganization of different actin structures in cells. The actin cytoskeleton is responsible for building a diverse array of structures with multiple functions, ranging from contractile actin/myosin filament bundles in skeletal and heart muscle to the very transient actin accumulations accompanying endo- or phagocytosis or the dynamic protrusive organelles such as lamellipodia and filopodia. Moreover, specific types of cell-cell or cell-substrate adhesions are frequently built and regulated by the actin cytoskeleton, and have decisive functions for normal physiology and disease, such as the invadopodia of cancer cells. Actin-dependent motility processes are equally important for effective immune responses and metastasis in cancer. In addition, the actin cytoskeleton is frequently usurped by viral or bacterial pathogens, in order to invade into or hide and spread within their host cells. We are thus mostly focussing on the molecular mechanisms of actin filament assembly and reorganization, especially at the plasma membrane, and how these processes are abused by bacterial pathogens.
Actin filament assembly involves both nucleation and elongation. In the meantime, the field distinguishes between three classes of nucleators: (i) Arp2/3 complex and activators, (ii) the family of formins and (iii) the third class comprising Spir, Cobl and Leiomodin.
The Arp2/3-complex harbours seven subunits, including the two actin-related proteins Arp2 and Arp3. The complex is mostly inactive in the absence of so called nucleation promoting factors (NPFs), the founding member of which is known as WASP, mutated in the rare, X-linked immunodeficiency Wiskott Aldrich Syndrome. The family in mammals comprises as classical members haematopoietic WASP, the more ubiquitous and neuronally enriched N-WASP and three WAVE isoforms (1,2,3). More recently, additional family members have appeared on the scene, termed WASH, WHAMM and JMY. The group of the eight activators of Arp2/3 complex are also known as type I nucleation promoting factors (NPFs).
In addition, WASP/N-WASP and WAVEs are direct or indirect effectors of small Rho (Ras homology) - family GTPases, prominent members of which are able to stimulate actin-based cell protrusions. It is generally agreed today that small GTPases of the Rac subfamily drive lamellipodia protrusion by connecting to WAVE mediating Arp2/3 complex activation. In spite of the commonly accepted, essential function of Rac/WAVE and Arp2/3 complex for lamellipodial actin filament assembly, more recent work reveals an additional contribution of other Rho-GTPase signalling pathways. For instance, we were recently able to show how Cdc42 supports the protrusion of lamellipodia through activation of the formin family members FMNL2 and FMNL3.
Another group of Arp2/3 complex interactors is called type II NPFs, and in mammals comprises the Src-substrate cortactin and its haematopoietic counterpart HS1. Both proteins accumulate at sites of dynamic actin assembly, and show a great degree of co-localization in cells with Arp2/3-complex. In our group, we have generated a murine, conditional cortactin KO mutant, which – in combination with a previously published conventional HS1 mutant model - allows dissecting cortactin/HS1 functions in specific processes and tissues.
In the recent past, we have also begun to dissect aforementioned signalling pathways, for instance those stimulated by the small Rho-family GTPase Rac and its relatives RhoG and Cdc42, by employing CRISPR/Cas9-mediated genome editing. In these efforts, selective removal of individual components within these signalling pathways is increasingly complemented by their replacement with variants harbouring distinct, unique activities, enabling new levels of insight into and comprehension of these signalling pathways. We propose that systematic analyses of that kind – certainly combined with mathematical modelling of connected changes of biochemical activities of analysed components and networks in different experimental conditions – will pave the way for the development of novel models for the modes of action of actin structures engaged in motility processes and specific host-pathogen interactions.
Our work benefits from numerous, fruitful national and international collaborations, for instance with the labs of
- Jan Faix (Hannover),
- Cord Brakebusch (Kopenhagen, Denmark),
- Michael Sixt (Klosterneuburg, Austria),
- Michael Schnoor (Mexico City) or
- Grégory Giannone (Bordeaux, France).
In various distinct projects, for instance funded by the Deutsche Forschungsgemeinschaft (DFG), molecular manipulations in cells are combined with high resolution imaging and biophysical measurements or mathematical modelling (Martin Falcke, Berlin). Both at TU Braunschweig (Biocenter and BRICS) and at HZI, we are currently establishing and/or extending on already established collaborations, for instance with HZI-departments Structure and Function of Proteins (Wulf Blankenfeldt), Cell Biology (Theresia Stradal) or Research Groups ZEIM (Manfred Rohde) and CPRO (Lothar Jänsch), as well as at TU with Martin Korte and Kristin Michaelsen-Preusse (Zoological Institute) or Peter Jomo Walla (Institute for Physical and Theoretical Chemistry).
Our Research
Our group is mainly interested in understanding the molecular mechanisms that drive the reorganization of different actin structures in cells. The actin cytoskeleton is responsible for building a diverse array of structures with multiple functions, ranging from contractile actin/myosin filament bundles in skeletal and heart muscle to the very transient actin accumulations accompanying endo- or phagocytosis or the dynamic protrusive organelles such as lamellipodia and filopodia. Moreover, specific types of cell-cell or cell-substrate adhesions are frequently built and regulated by the actin cytoskeleton, and have decisive functions for normal physiology and disease, such as the invadopodia of cancer cells. Actin-dependent motility processes are equally important for effective immune responses and metastasis in cancer. In addition, the actin cytoskeleton is frequently usurped by viral or bacterial pathogens, in order to invade into or hide and spread within their host cells. We are thus mostly focussing on the molecular mechanisms of actin filament assembly and reorganization, especially at the plasma membrane, and how these processes are abused by bacterial pathogens.
Actin filament assembly involves both nucleation and elongation. In the meantime, the field distinguishes between three classes of nucleators: (i) Arp2/3 complex and activators, (ii) the family of formins and (iii) the third class comprising Spir, Cobl and Leiomodin.
The Arp2/3-complex harbours seven subunits, including the two actin-related proteins Arp2 and Arp3. The complex is mostly inactive in the absence of so called nucleation promoting factors (NPFs), the founding member of which is known as WASP, mutated in the rare, X-linked immunodeficiency Wiskott Aldrich Syndrome. The family in mammals comprises as classical members haematopoietic WASP, the more ubiquitous and neuronally enriched N-WASP and three WAVE isoforms (1,2,3). More recently, additional family members have appeared on the scene, termed WASH, WHAMM and JMY. The group of the eight activators of Arp2/3 complex are also known as type I nucleation promoting factors (NPFs).
In addition, WASP/N-WASP and WAVEs are direct or indirect effectors of small Rho (Ras homology) - family GTPases, prominent members of which are able to stimulate actin-based cell protrusions. It is generally agreed today that small GTPases of the Rac subfamily drive lamellipodia protrusion by connecting to WAVE mediating Arp2/3 complex activation. In spite of the commonly accepted, essential function of Rac/WAVE and Arp2/3 complex for lamellipodial actin filament assembly, more recent work reveals an additional contribution of other Rho-GTPase signalling pathways. For instance, we were recently able to show how Cdc42 supports the protrusion of lamellipodia through activation of the formin family members FMNL2 and FMNL3.
Another group of Arp2/3 complex interactors is called type II NPFs, and in mammals comprises the Src-substrate cortactin and its haematopoietic counterpart HS1. Both proteins accumulate at sites of dynamic actin assembly, and show a great degree of co-localization in cells with Arp2/3-complex. In our group, we have generated a murine, conditional cortactin KO mutant, which – in combination with a previously published conventional HS1 mutant model - allows dissecting cortactin/HS1 functions in specific processes and tissues.
In the recent past, we have also begun to dissect aforementioned signalling pathways, for instance those stimulated by the small Rho-family GTPase Rac and its relatives RhoG and Cdc42, by employing CRISPR/Cas9-mediated genome editing. In these efforts, selective removal of individual components within these signalling pathways is increasingly complemented by their replacement with variants harbouring distinct, unique activities, enabling new levels of insight into and comprehension of these signalling pathways. We propose that systematic analyses of that kind – certainly combined with mathematical modelling of connected changes of biochemical activities of analysed components and networks in different experimental conditions – will pave the way for the development of novel models for the modes of action of actin structures engaged in motility processes and specific host-pathogen interactions.
Our work benefits from numerous, fruitful national and international collaborations, for instance with the labs of
- Jan Faix (Hannover),
- Cord Brakebusch (Kopenhagen, Denmark),
- Michael Sixt (Klosterneuburg, Austria),
- Michael Schnoor (Mexico City) or
- Grégory Giannone (Bordeaux, France).
In various distinct projects, for instance funded by the Deutsche Forschungsgemeinschaft (DFG), molecular manipulations in cells are combined with high resolution imaging and biophysical measurements or mathematical modelling (Martin Falcke, Berlin). Both at TU Braunschweig (Biocenter and BRICS) and at HZI, we are currently establishing and/or extending on already established collaborations, for instance with HZI-departments Structure and Function of Proteins (Wulf Blankenfeldt), Cell Biology (Theresia Stradal) or Research Groups ZEIM (Manfred Rohde) and CPRO (Lothar Jänsch), as well as at TU with Martin Korte and Kristin Michaelsen-Preusse (Zoological Institute) or Peter Jomo Walla (Institute for Physical and Theoretical Chemistry).
Prof. Dr. Klemens Rottner

Klemens Rottner studied Zoology/Biology at Paris-Lodron-University Salzburg and after graduation, he continued with his PhD studies in the field of Cell Biology/Immunology. In 2000, he moved to Braunschweig and worked as postdoc in the Department for Cell Biology at the formerly called German Research Centre for Biotechnology (GBF). In 2004, he became head of the research group Cytoskeleton Dynamics at the Helmholtz Centre for Infection Research. In 2010, he was called as professor and head of the Research Unit Actin Dynamics and Motility to the Rheinische Friedrich-Wilhelms-University in Bonn (Institute for Genetics), but returned to Braunschweig in 2014.
Since then he operates as professor at the Institute for Zoology at the TU Braunschweig and additionally heads the research group of Molecular Cell Biology at the Helmholtz-Centre for Infection Research.
Team






Selected Publications
Rottner, K., Stradal, T.E.B., Chen, B. (2021) WAVE regulatory complex. Curr Biol, 31(10):R512-R517. DOI: 10.1016/j.cub.2021.01.086.
Dimchev, V., Lahmann, I., Koestler, S.A., Kage, F., Dimchev, G., Steffen, A., Stradal, T.E.B., Vauti, F., Arnold, H.H., Rottner, K. (2021) Induced Arp2/3 complex depletion increases FMNL2/3 formin expression and filopodia formation. Front Cell Dev Biol, 9:634708. DOI: 10.3389/fcell.2021.634708.
Dimchev, G., Amiri, B., Humphries, A.C., Schaks, M., Dimchev, V., Stradal, T.E., Faix, J., Krause, M., Way, M., Falcke, M., Rottner, K. (2020) Lamellipodin tunes cell migration by stabilizing protrusions and promoting adhesion formation. J Cell Sci, 133(7):jcs239020.
Schaks, M., Singh, S.P., Kage, F., Thomason, P., Klünemann, T., Steffen, A., Blankenfeldt, W., Stradal, T.E., Insall, R., Rottner, K. (2018) Distinct interaction sites of Rac GTPase with WAVE regulatory complex have non-redundant functions in vivo. Curr Biol, 28(22):3674-3684. doi: 10.1016/j.cub.2018.10.002.
Kage, F., Winterhoff, M., Dimchev, V., Mueller, J., Thalheim, T., Freise, A., Brühmann, S., Kollasser, J., Block, J., Dimchev, G., Geyer, M., Schnittler, H.-J., Brakebusch, C., Stradal, T.E., Carlier, M.-F., Sixt, M., Käs, J., Faix, J., Rottner, K. (2017) FMNL formins boost lamellipodial force generation. Nat Commun, 8:14832.
Publications
Ongoing Projects
Dissection of formin functions in cell edge protrusion and migration
Partners:
- Prof. Dr. Jan Faix (Hannover)
- Prof. Dr. Michael Sixt (Klosterneuburg, Austria)
- Dr. Florian Schur (Klosterneuburg, Austria)
Funding Agency:
- DFG - German Research Foundation
Project P4: Lamellipodial protein complexes driving Arp2/3-dependent branching (PROCOMPAS)
Funding Agency:
- DFG - German Research Foundation